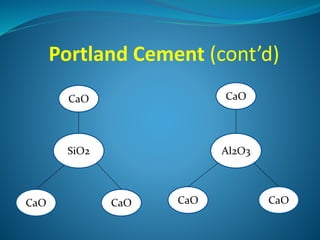
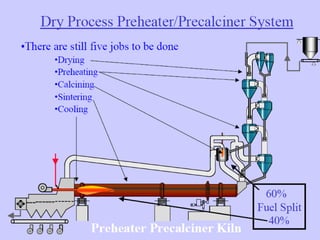
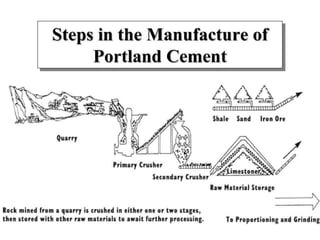
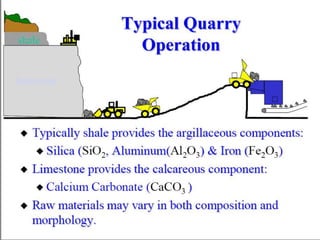
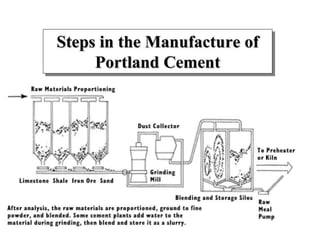
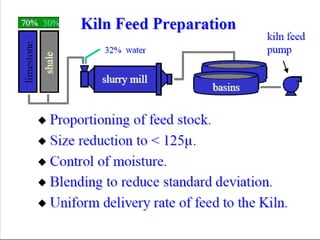
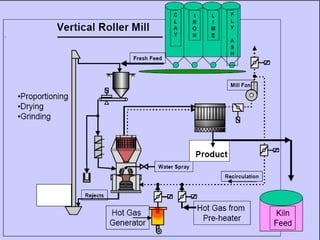
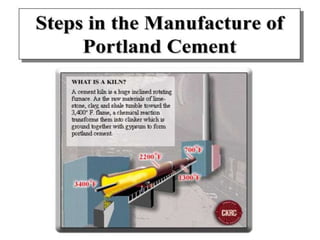
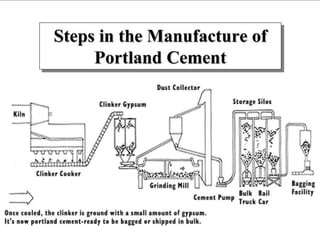
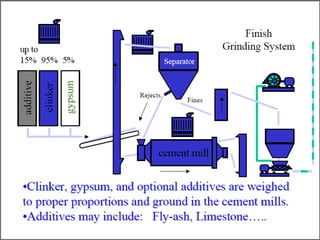
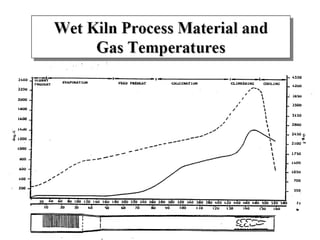
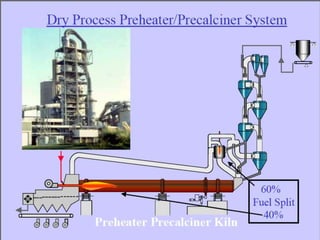
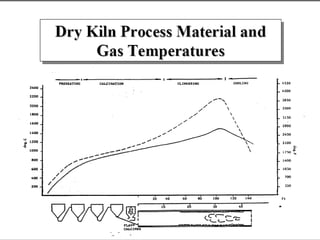
This document discusses Portland cement and the cement manufacturing process. It begins with an overview of what cement is and how it is used to make concrete. It then describes the industrial process for manufacturing cement, involving grinding raw materials like limestone and clay at high temperatures in a kiln to form clinker, which is then pulverized with gypsum to become Portland cement powder. The document also provides a brief history of cement development and explains how cement kilns can beneficially reuse solid and hazardous wastes as a source of energy and raw material replacement due to the kilns' high temperatures and long retention times.