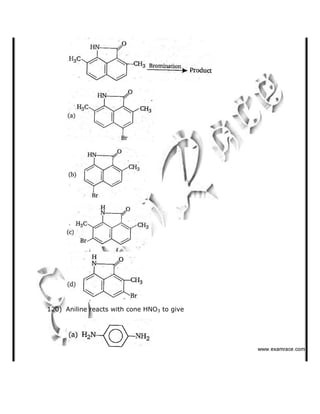
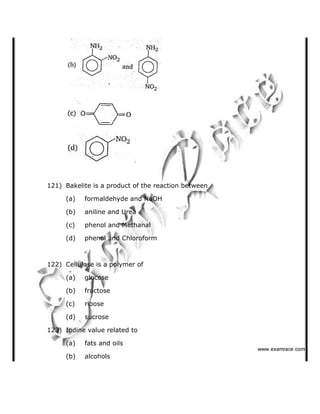
This document contains a solved practice paper for BITSAT entrance exam from 2007. It includes 76 multiple choice questions across subjects like mathematics, physics, chemistry. The questions range in difficulty from basic to advanced concepts. Sample questions include trigonometric identities, Newton's laws of motion, chemical bonding, gas laws, and more. The document provides questions, answer options, and the correct answer key to help students prepare for the BITSAT exam.









![(b) CuF2
(c) MgF2
(d) CuCl
116) The effective atomic number of Cr (at no = 24) in [Cr(NH3)6] Cl3
is
(a) 35
(b) 27
(c) 33
(d) 36
117) In Nessler's reagent for the detection of ammonia the active
species is
(a) Hg2Cl2
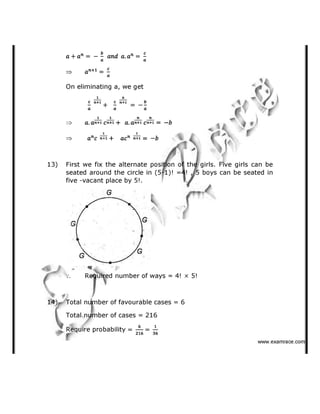
(b) Mg2+
(c) Hg2l2
(d) HgI4
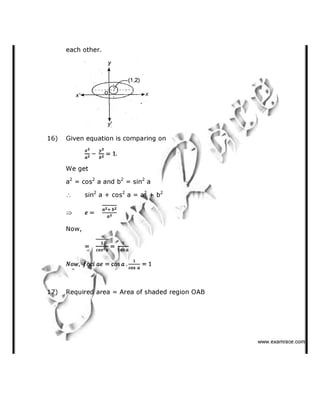
2-
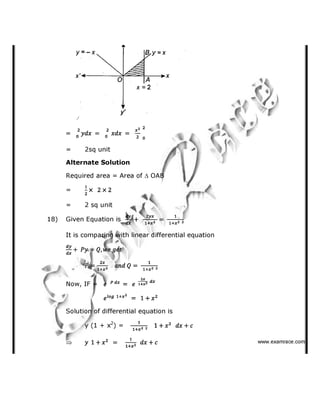
118) Which of the following ketones will not respond to iodoform test?
(a) Methyl isopropyl ketone
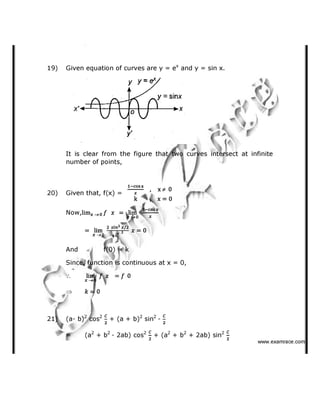
(b) Ethyl isopropyl ketone
(c) Dimethyl ketone
(d) 2-hexanone
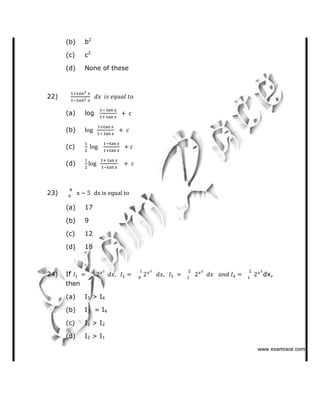
119) ✁✂✄☎✆✝☎✞✂✁✞✟✆](https://image.slidesharecdn.com/bitsat2007questionswithanswers-130802025237-phpapp01/85/Bitsat-2007-questions-with-answers-36-320.jpg)