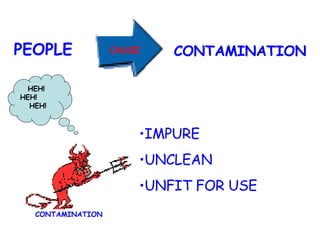
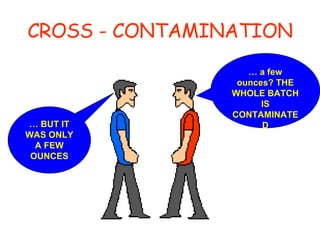
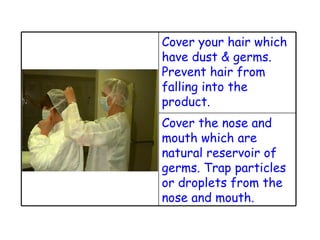
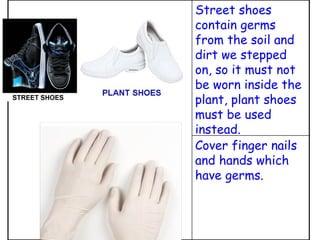
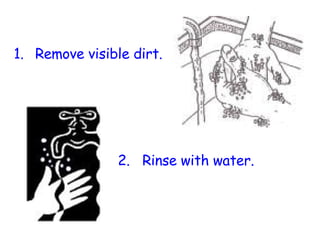
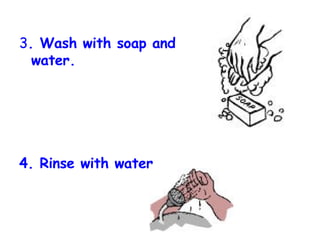
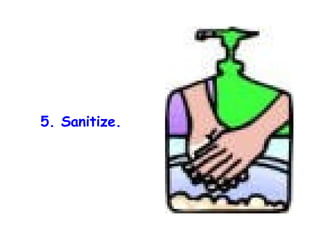
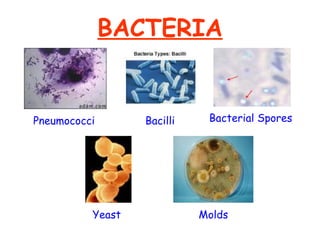
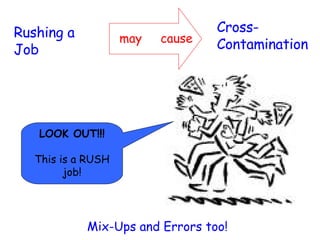
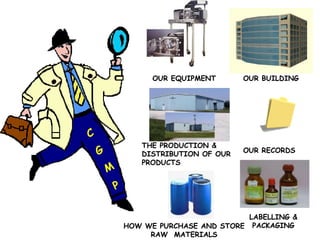
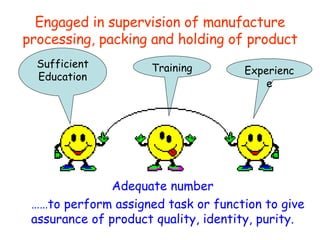
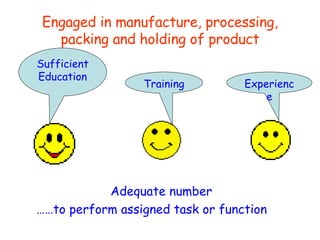
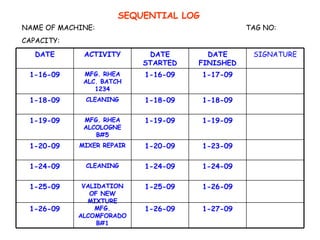
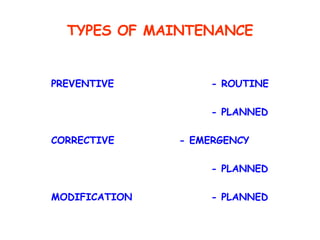
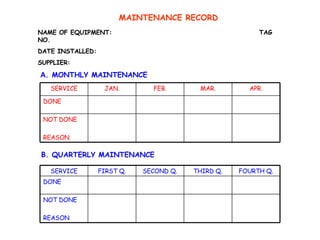
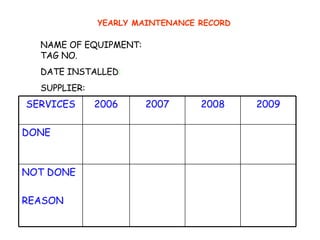
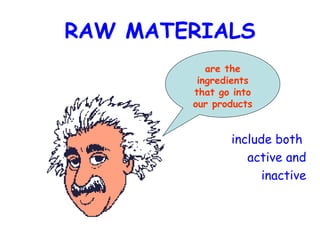
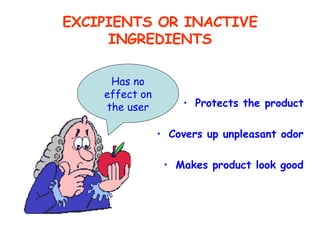
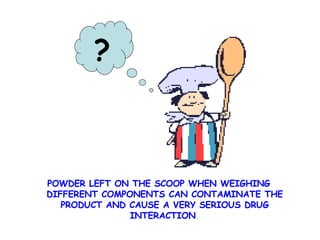
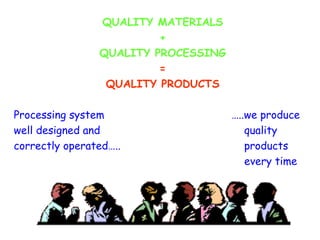
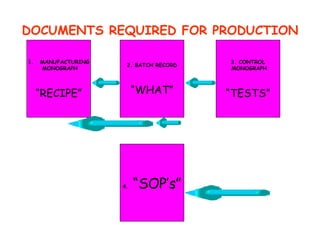
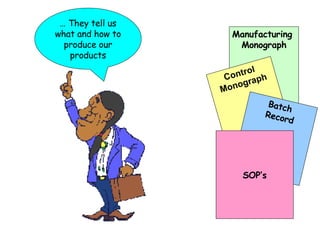
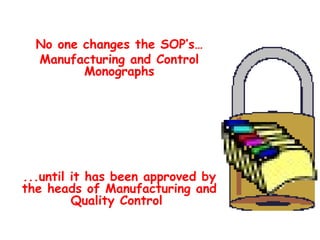
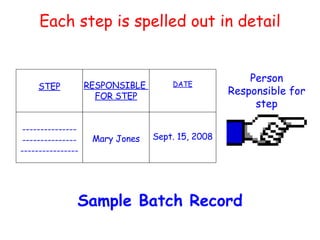
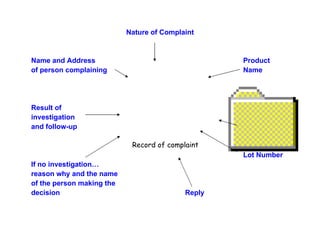
The document discusses Good Manufacturing Practices (GMP) and contamination prevention. It covers types of contamination, sources, and how to prevent them through practices like personal hygiene, sanitation, cleaning, and equipment maintenance. GMP regulations require facilities, equipment, personnel training, and documentation to help assure product quality and safety.