The document describes the structure of the nuclear atom according to the following key points:
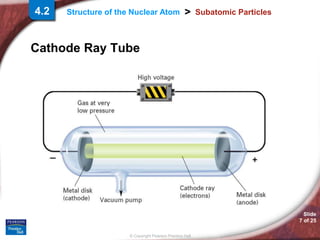
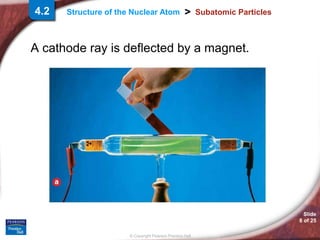
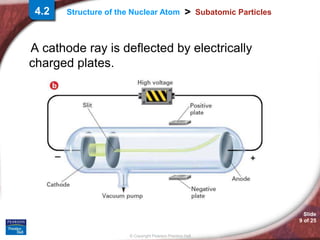
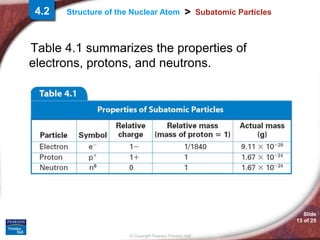
1) Subatomic particles like electrons, protons, and neutrons make up atoms. Experiments by J.J. Thomson, Eugen Goldstein, and James Chadwick discovered these particles.
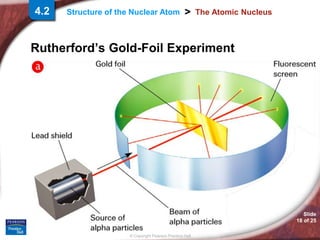
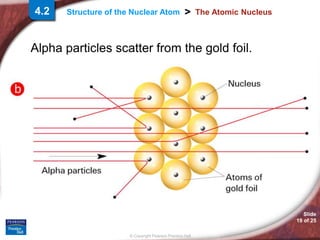
2) Ernest Rutherford's gold foil experiment showed that the mass and positive charge of atoms are concentrated in a tiny central nucleus, rather than being evenly distributed as previously thought.
3) The nucleus is composed of protons and neutrons, while electrons orbit the outer region of the atom. This Rutherford model depicts the basic structure of the nuclear atom.