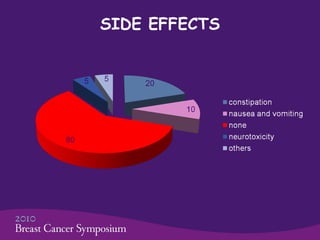
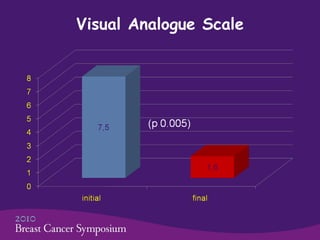
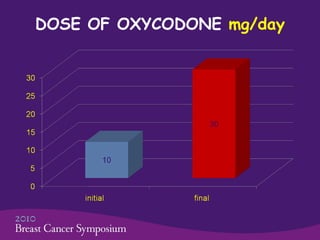
This phase II trial studied the use of oxycodone to treat refractory dyspnea in 20 advanced breast cancer patients. The average age was 59.5 years. Common side effects were constipation and nausea/vomiting. No patients stopped treatment due to side effects. Oxycodone was given at an average starting dose of 10 mg/day increasing to 30 mg/day over 75.5 days on average. Dyspnea scores improved significantly from an average of 7.5 initially to 1.6, showing oxycodone effectively controlled dyspnea. The study concluded oxycodone is effective and safe for dyspnea in these patients and should be compared to morphine in a phase III trial.