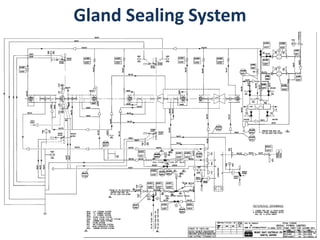
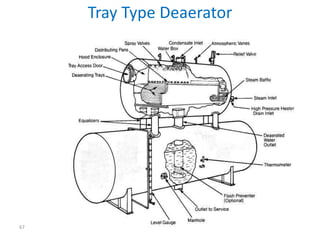
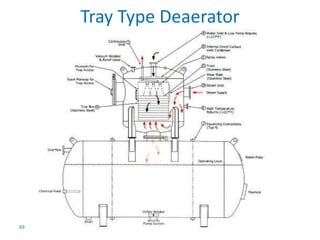
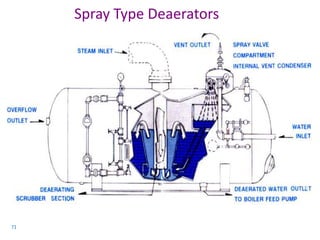
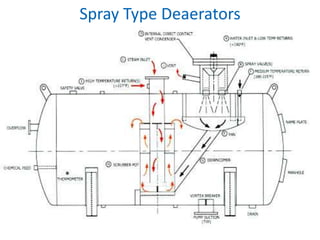
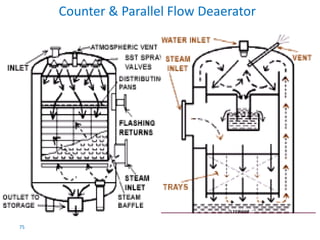
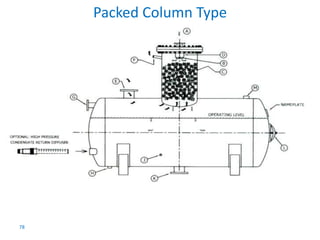
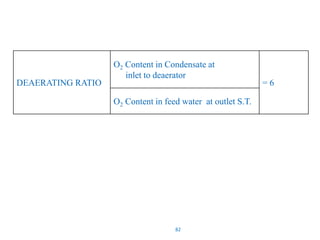
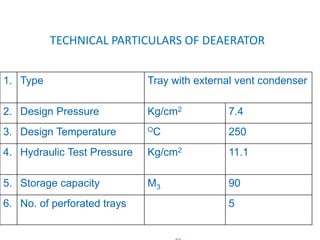
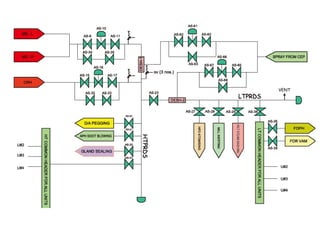
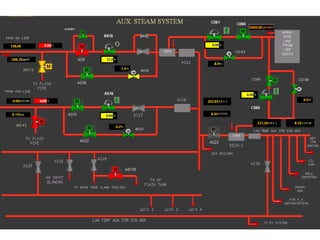
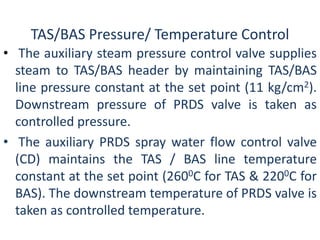
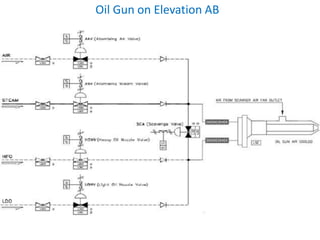
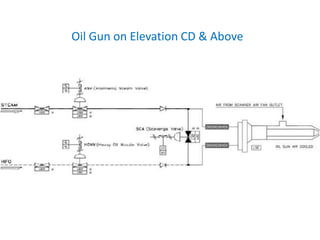
The document describes the auxiliary PRDS (pressure reducing and desuperheating) system used in thermal power plants. It has two identical systems - the turbine auxiliary steam system (TAS) and boiler auxiliary steam system (BAS). Low and high capacity auxiliary steam is derived from main steam and its pressure and temperature are reduced before supplying it to various locations in the plant for processes like deaeration, soot blowing, oil heating etc. The systems use control valves, isolating valves, desuperheaters and spray water to control pressure and temperature.





























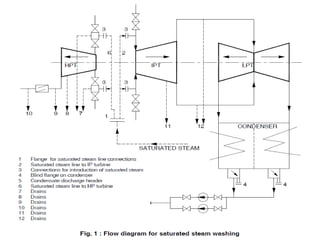
![Wet Steam Washing
• During operation, deposits occur on the turbine
blading to a greater or lesser degree depending on
the steam purity [1] and the pressures and
temperatures of the operating steam. These
deposits cause a reduction of the turbine generator
unit’s efficiency due to:
Changes in the flow profiles
• Thicker boundary layers in the steam
• flow as a result of rough surfaces.](https://image.slidesharecdn.com/aprdssystem-140907053421-phpapp02/85/Aprds-system-40-320.jpg)