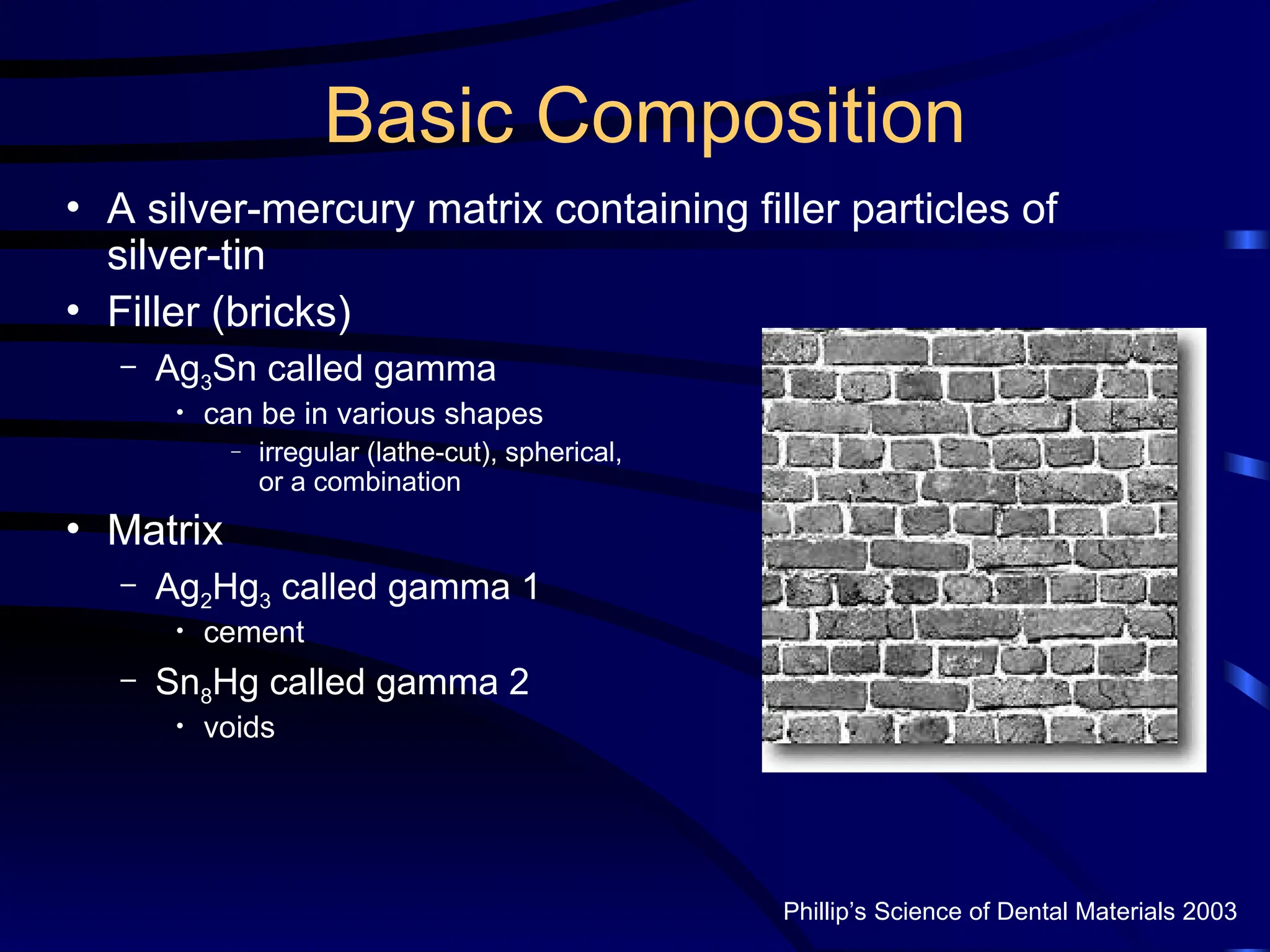
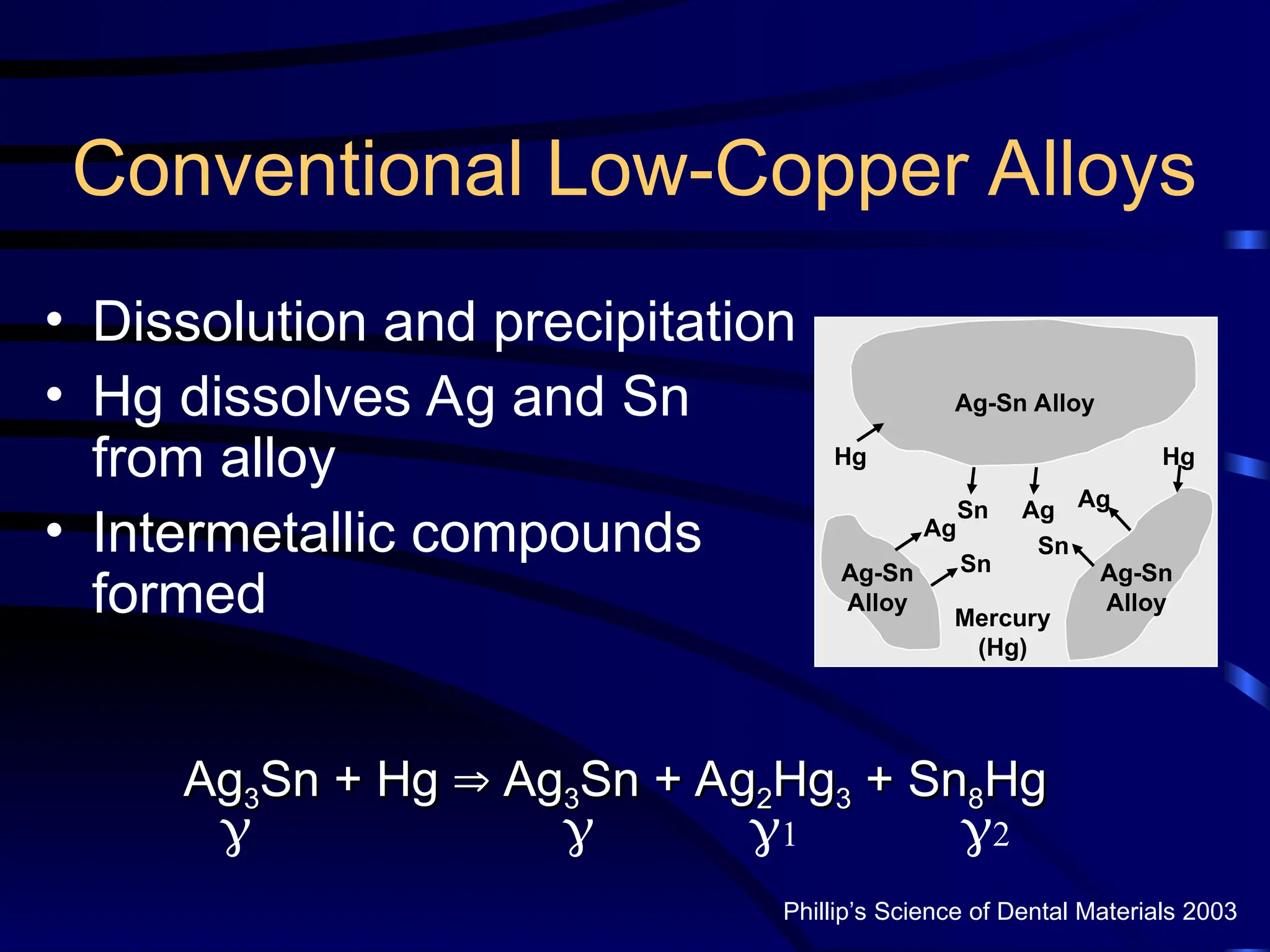
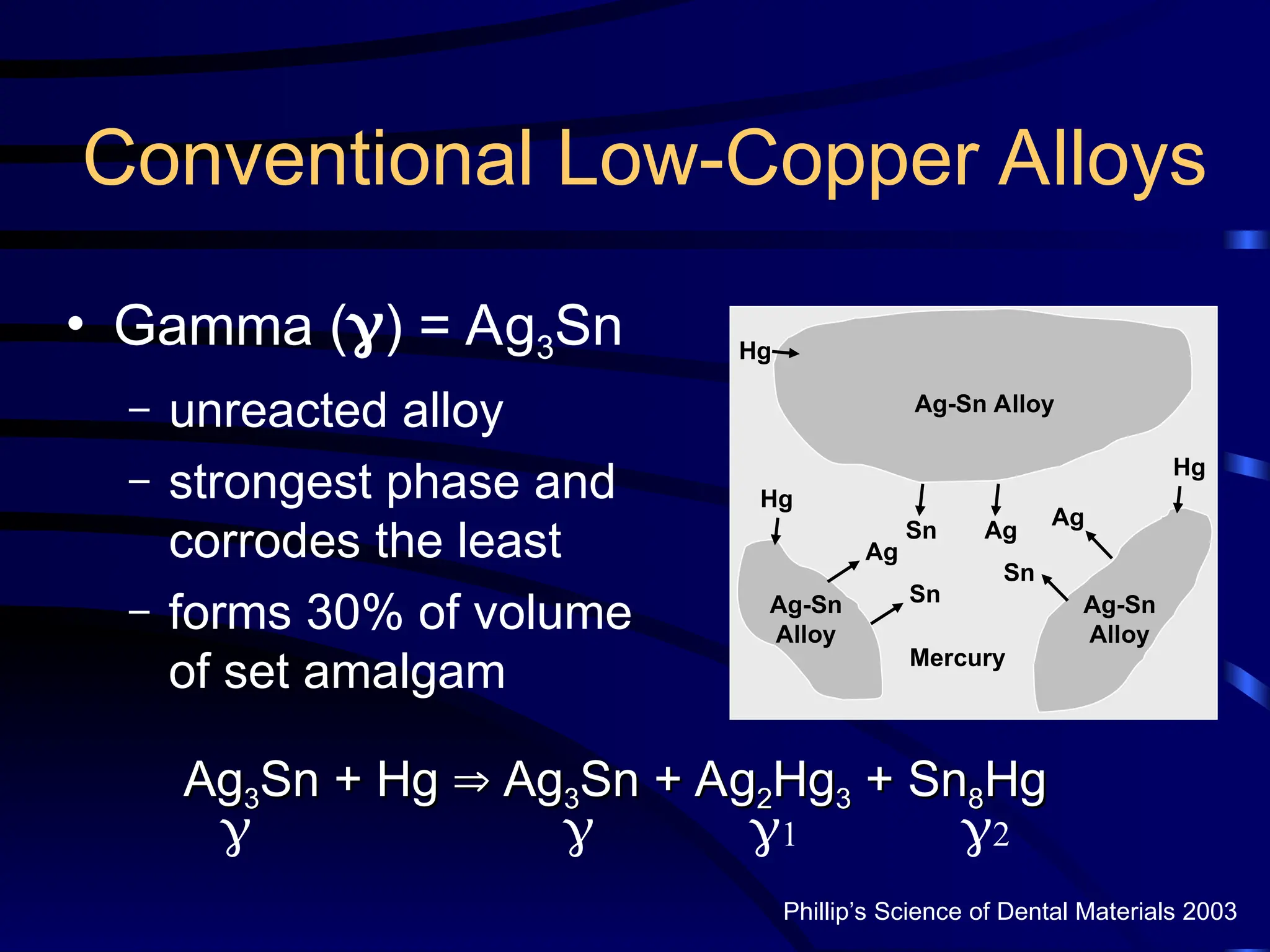
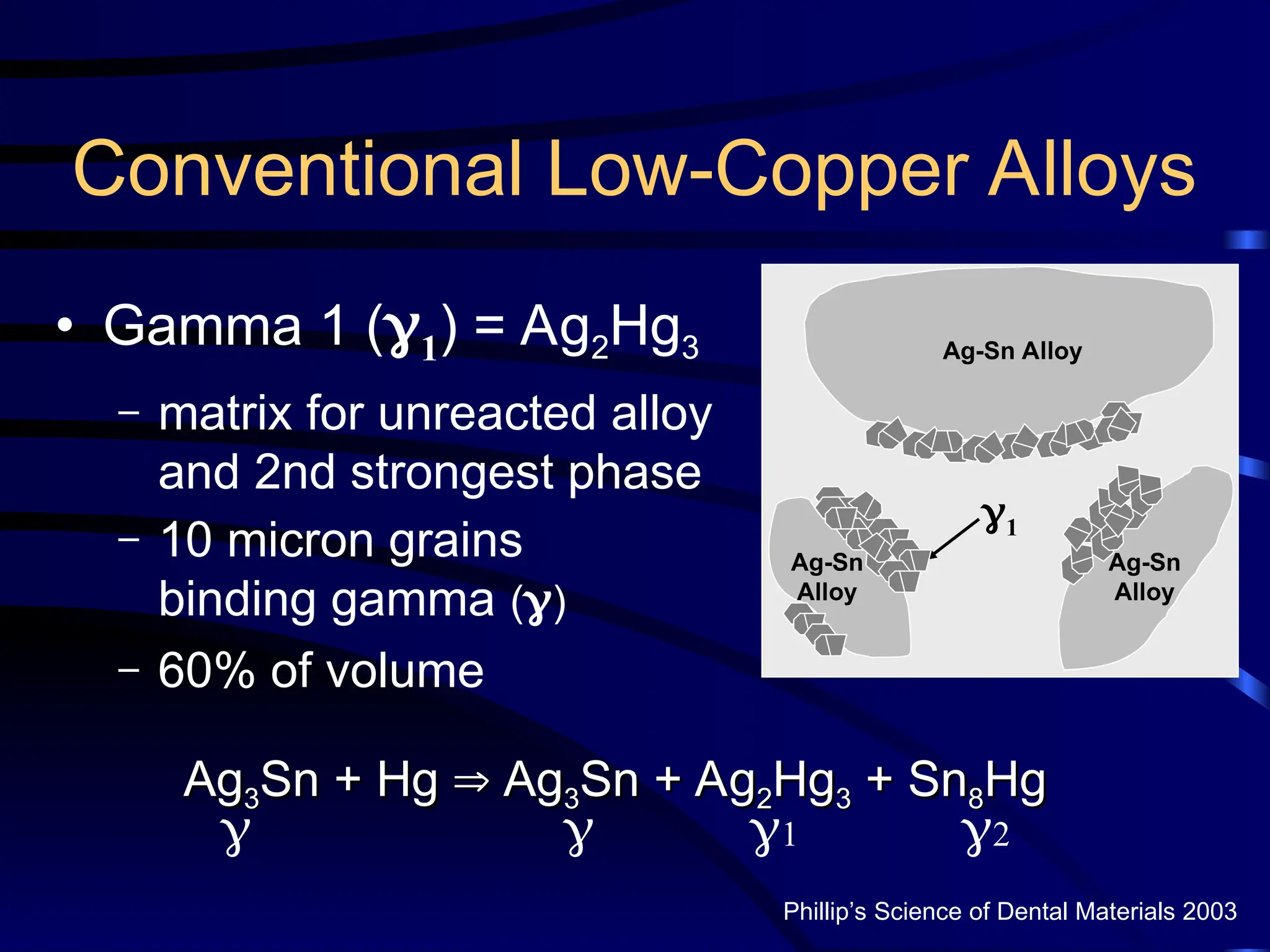
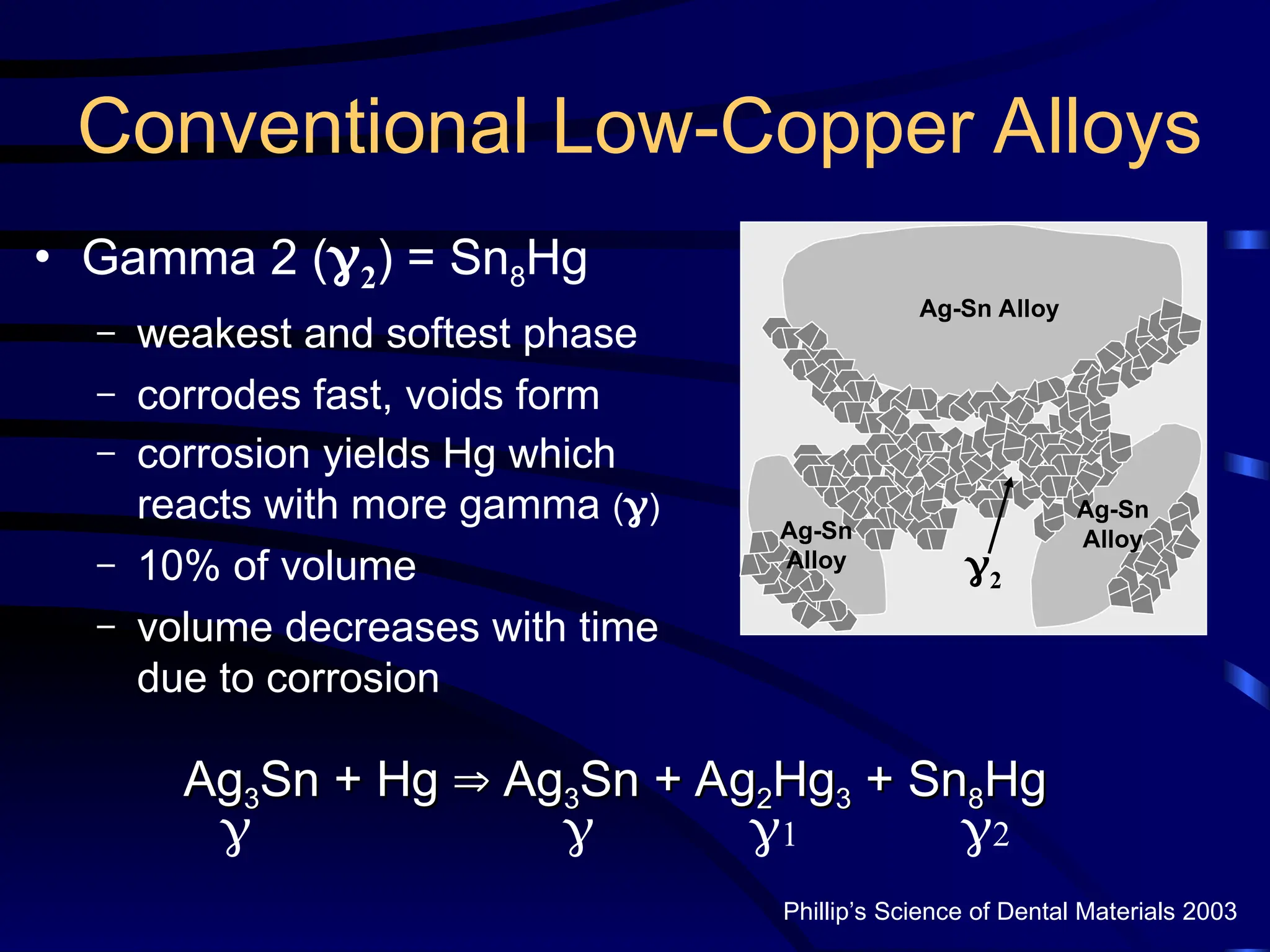
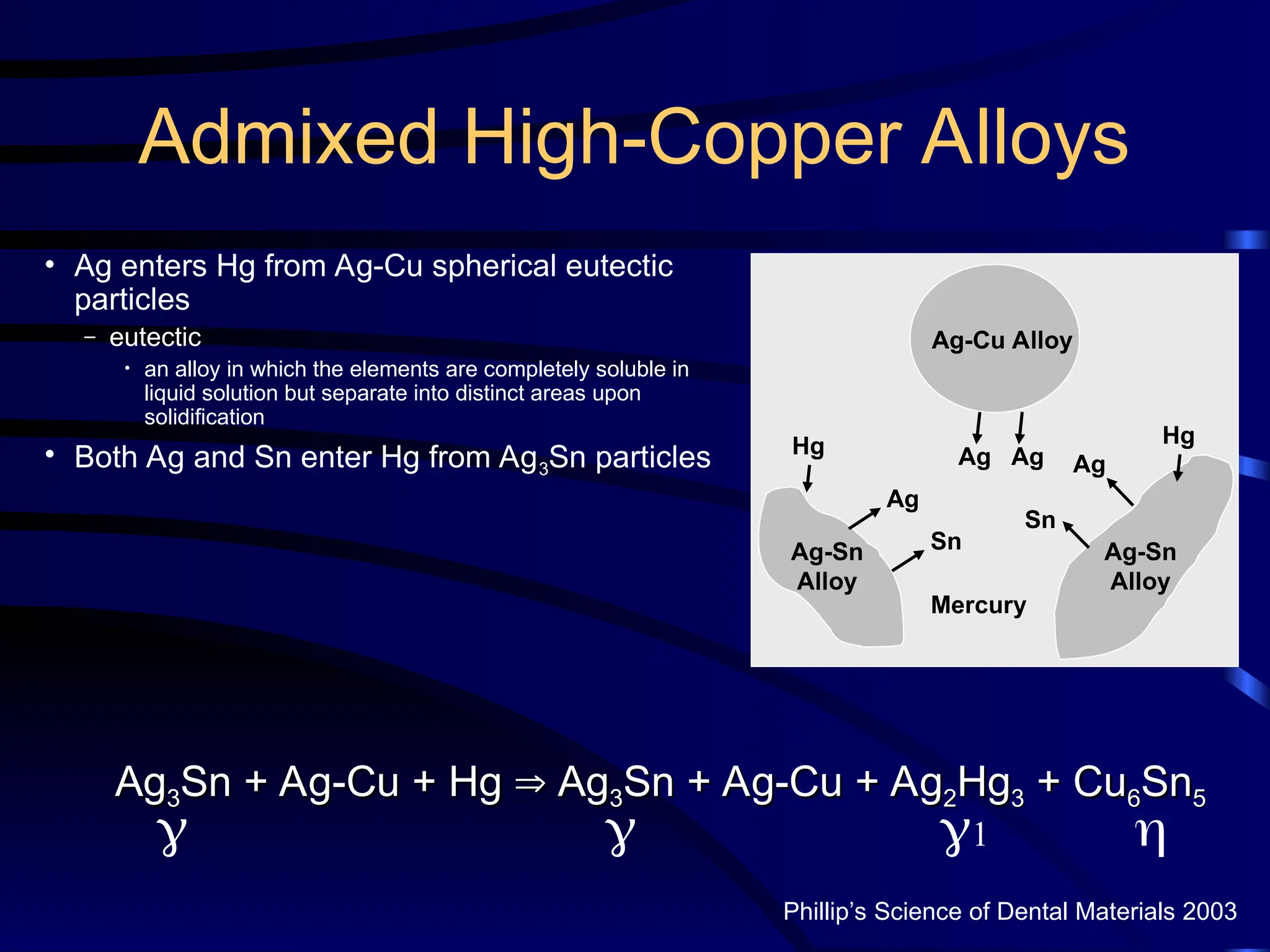
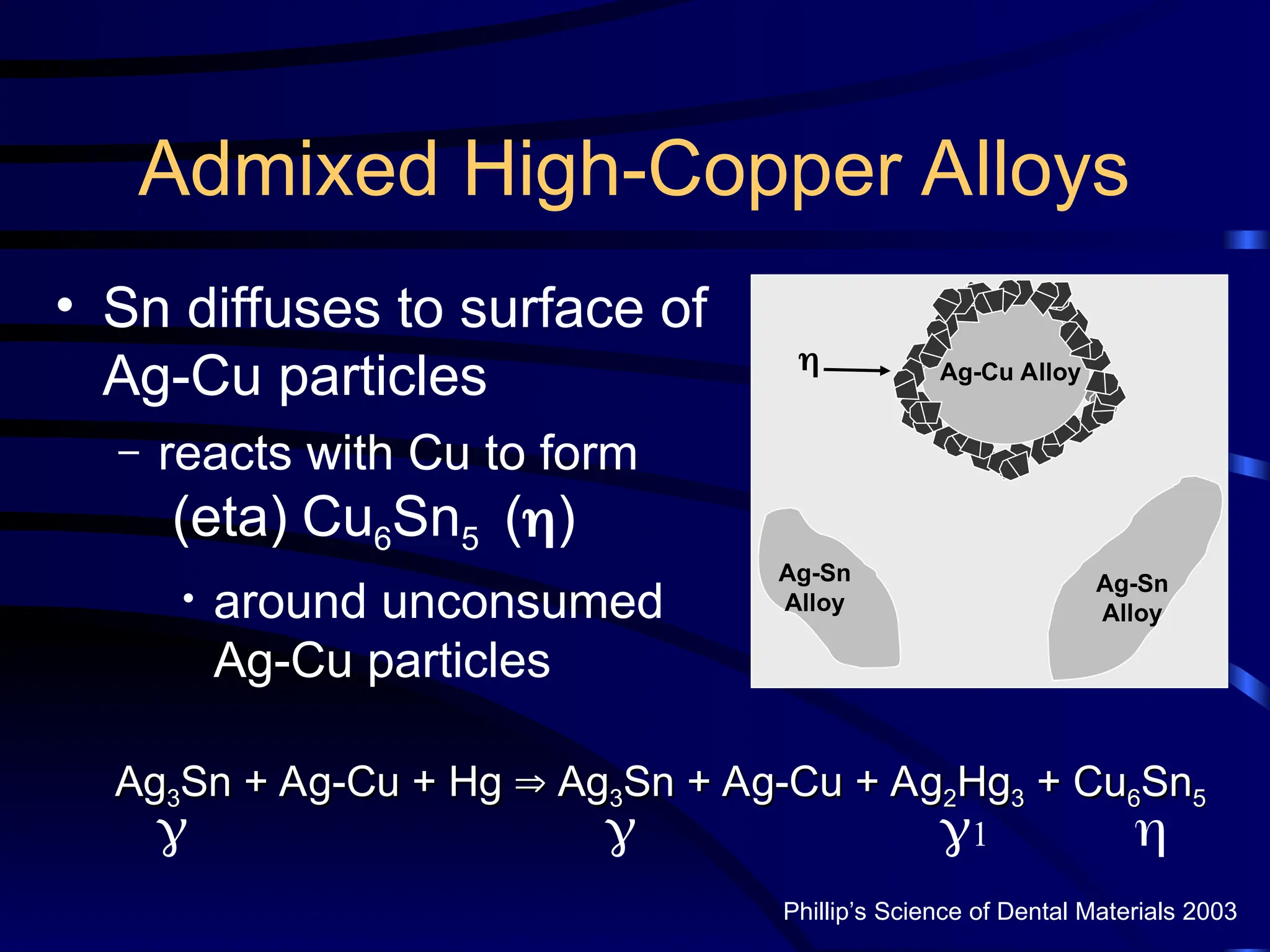
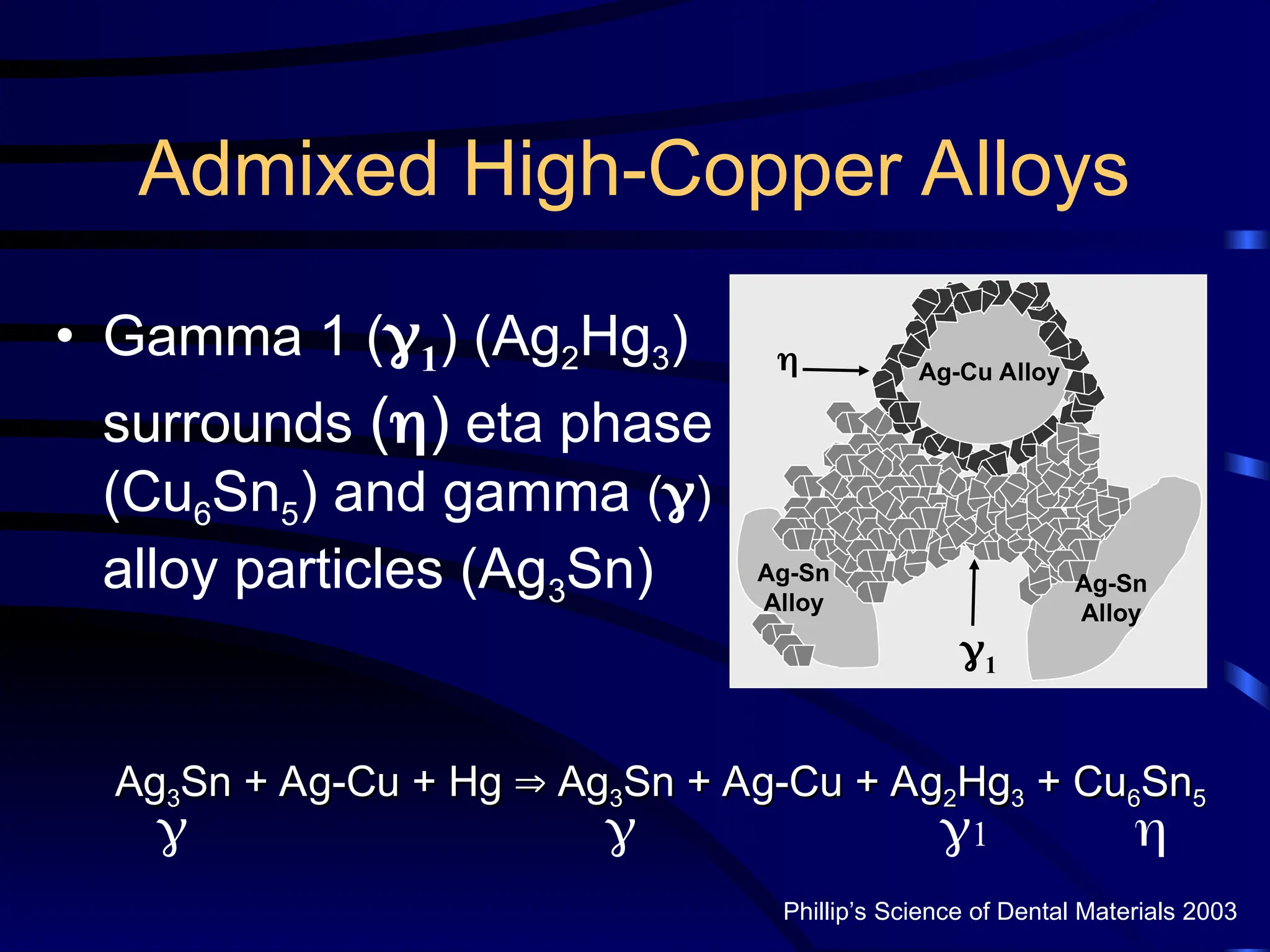
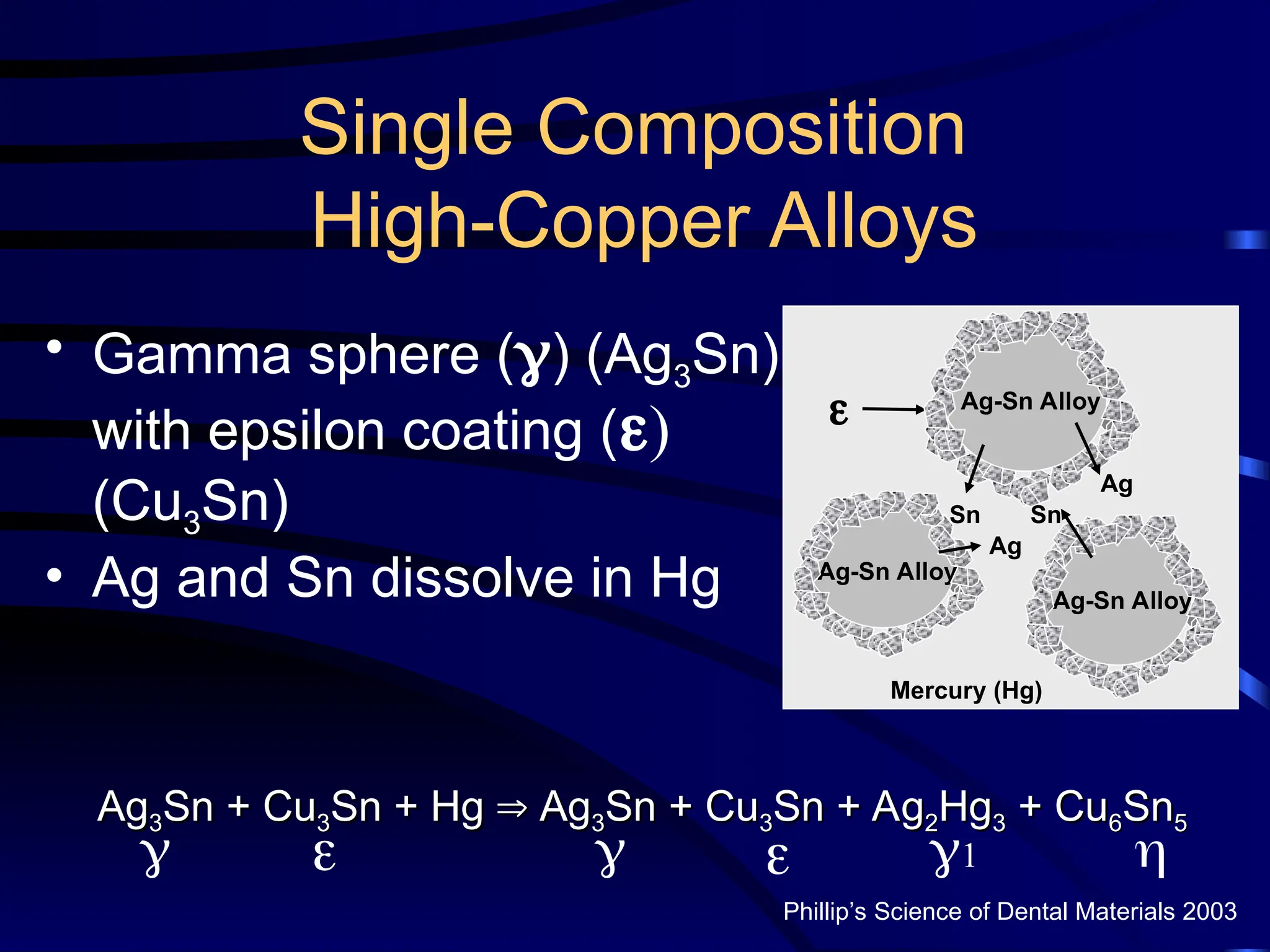
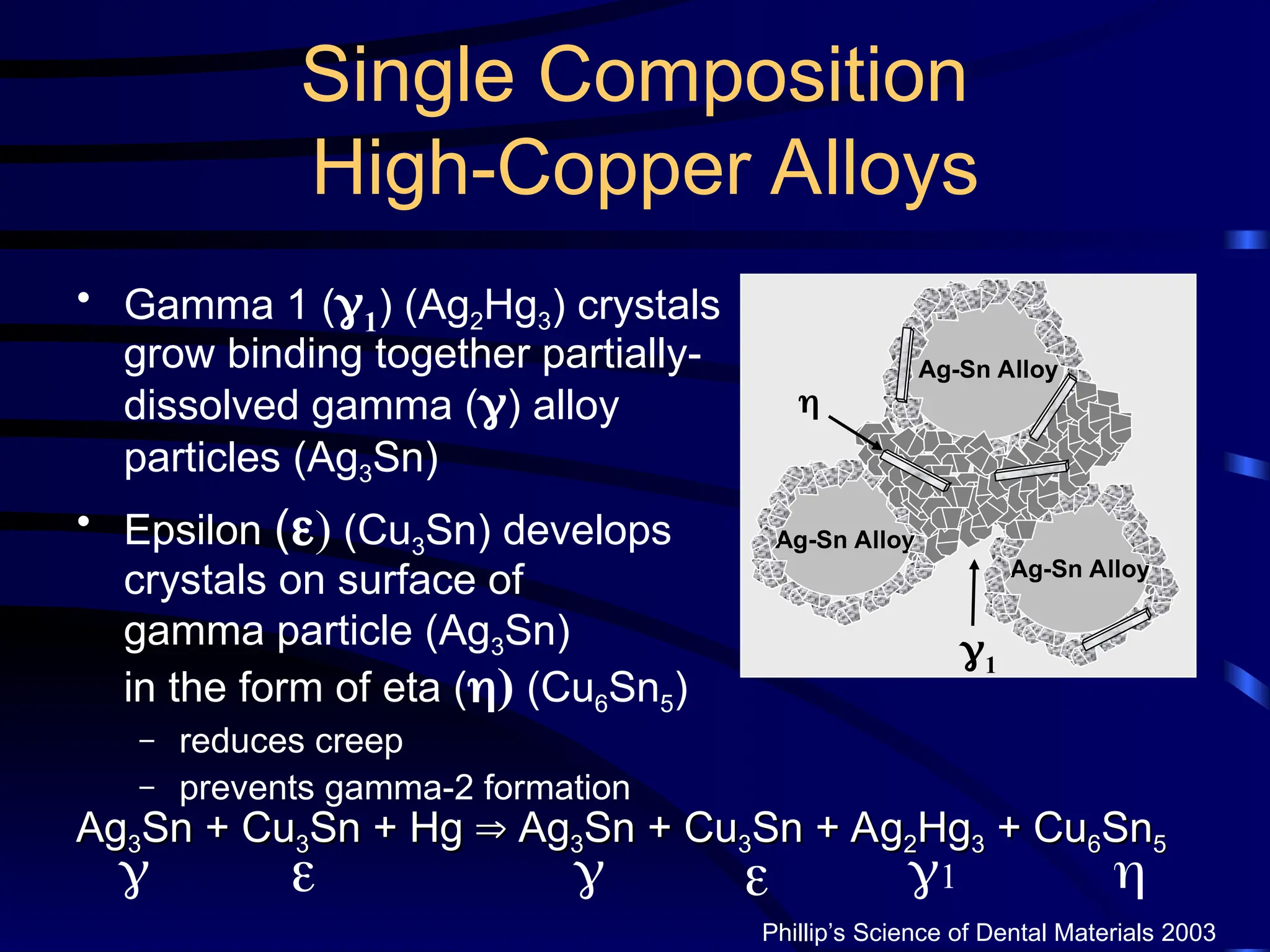
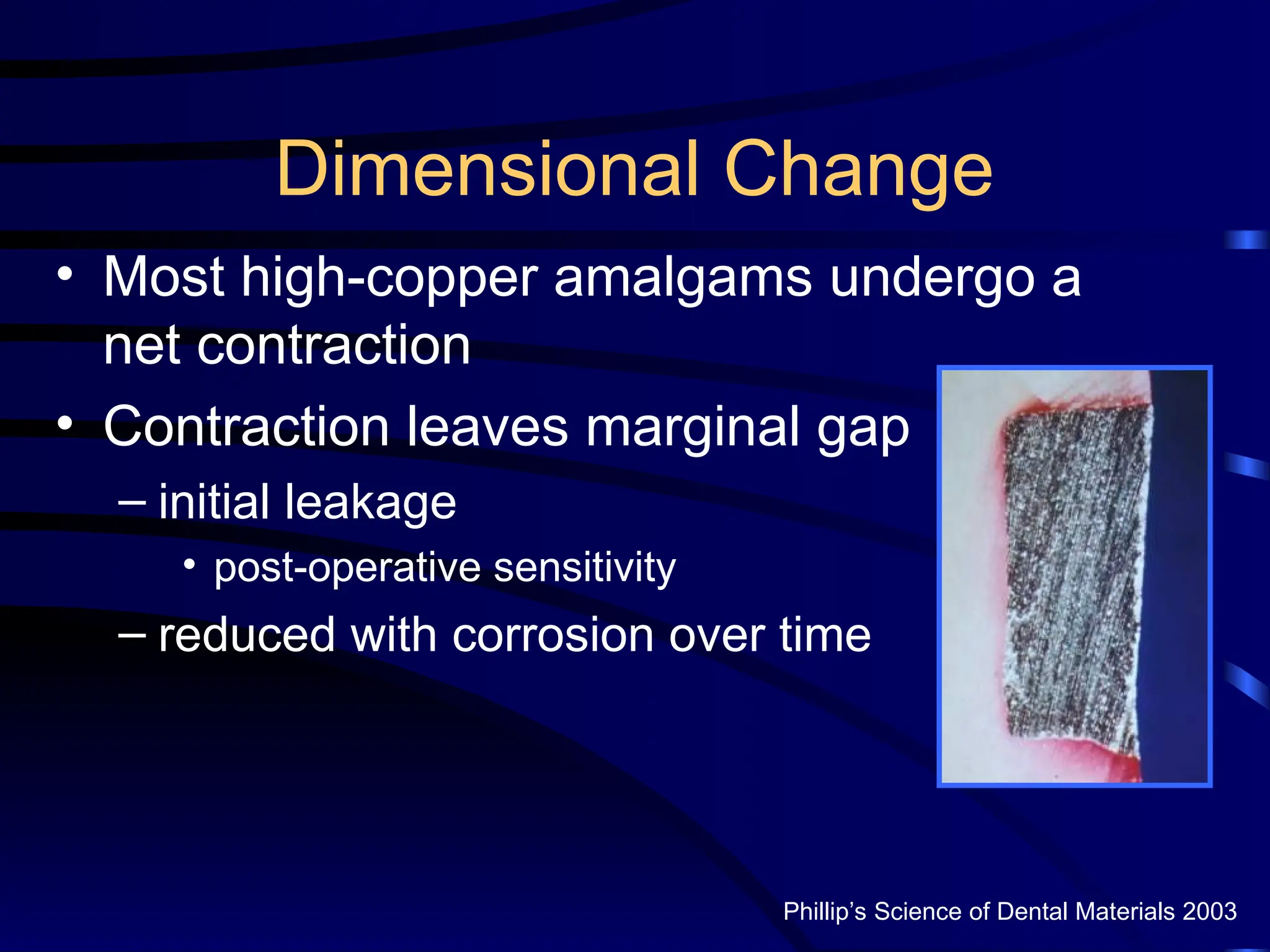
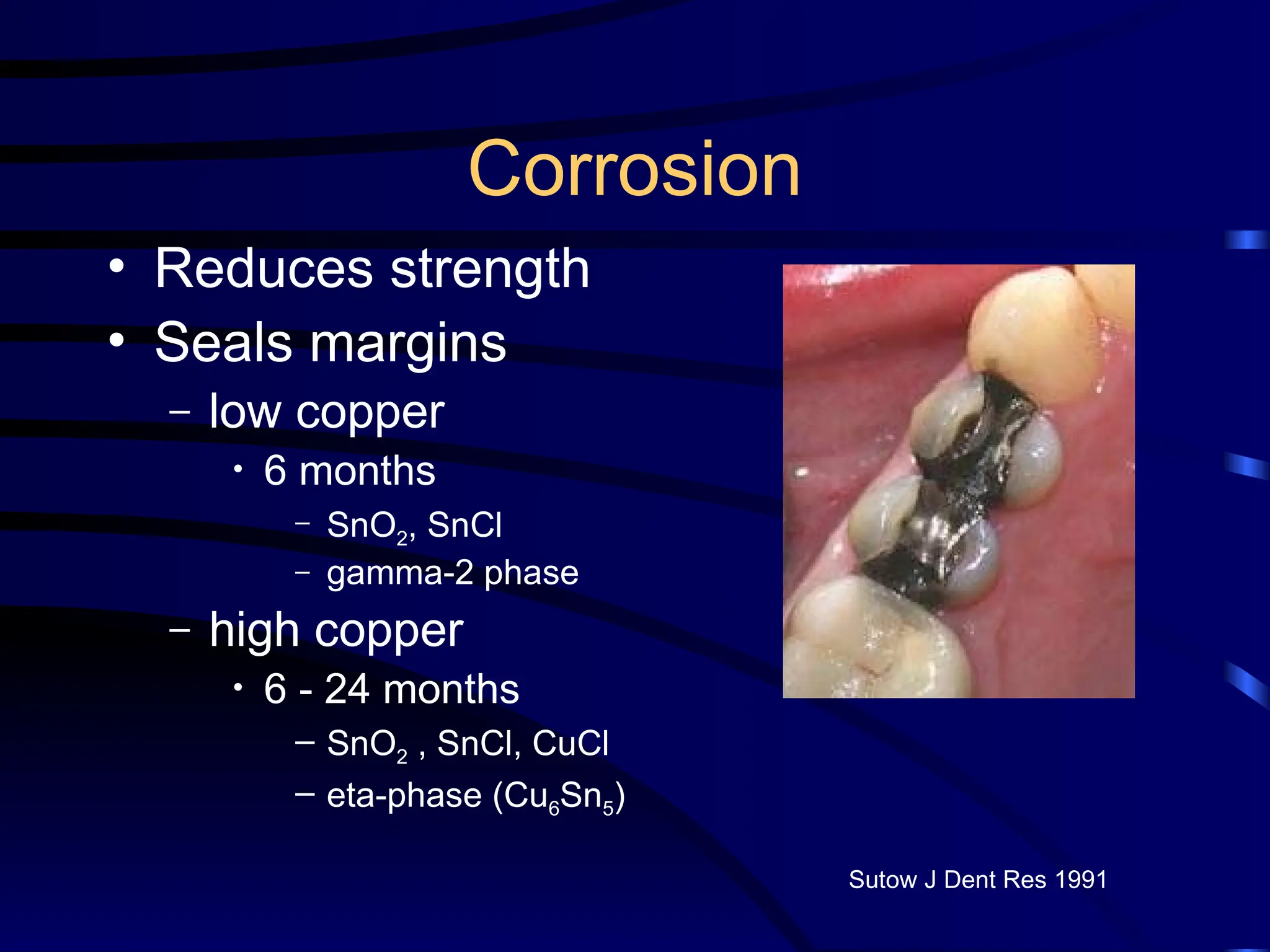
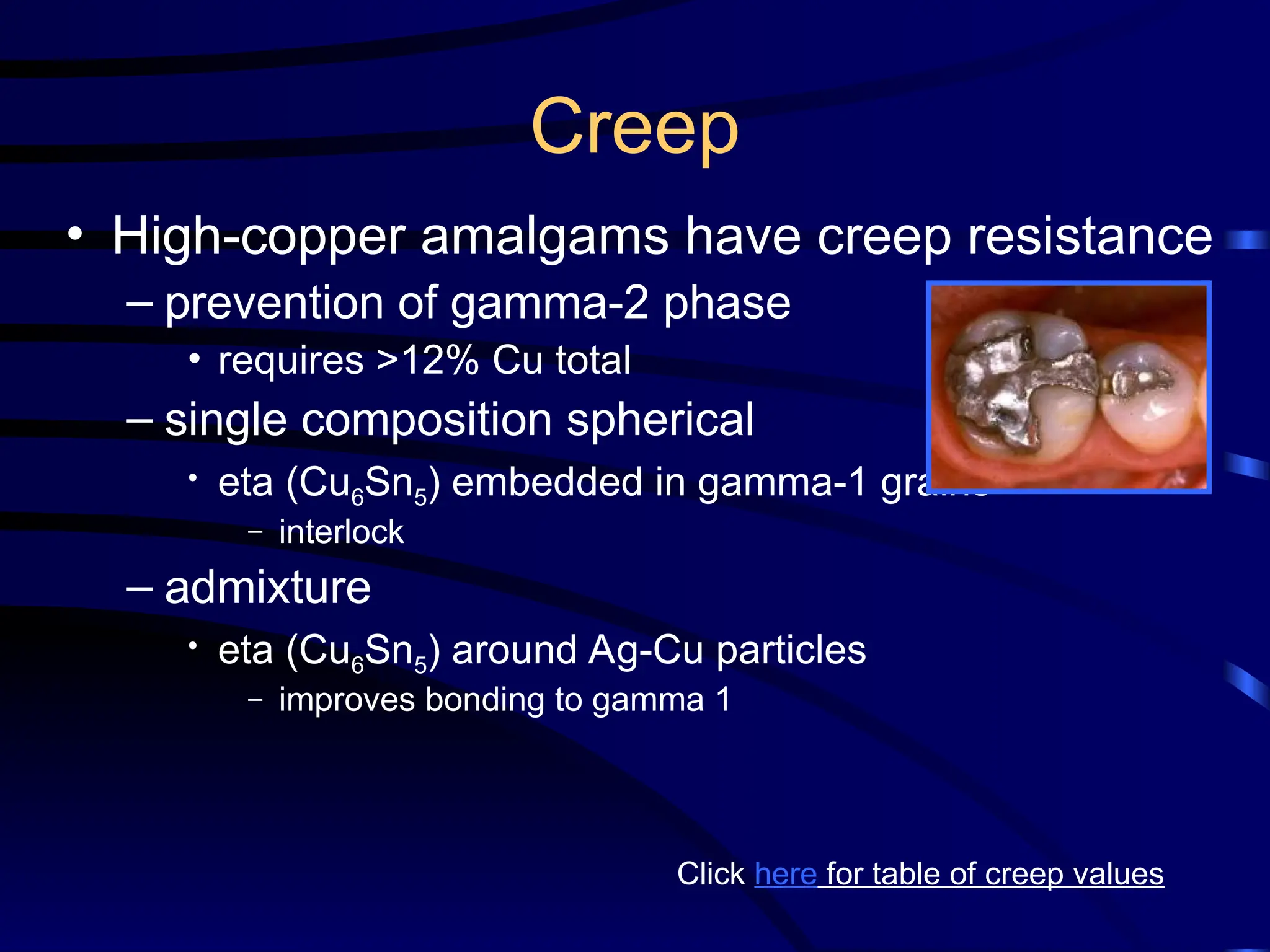
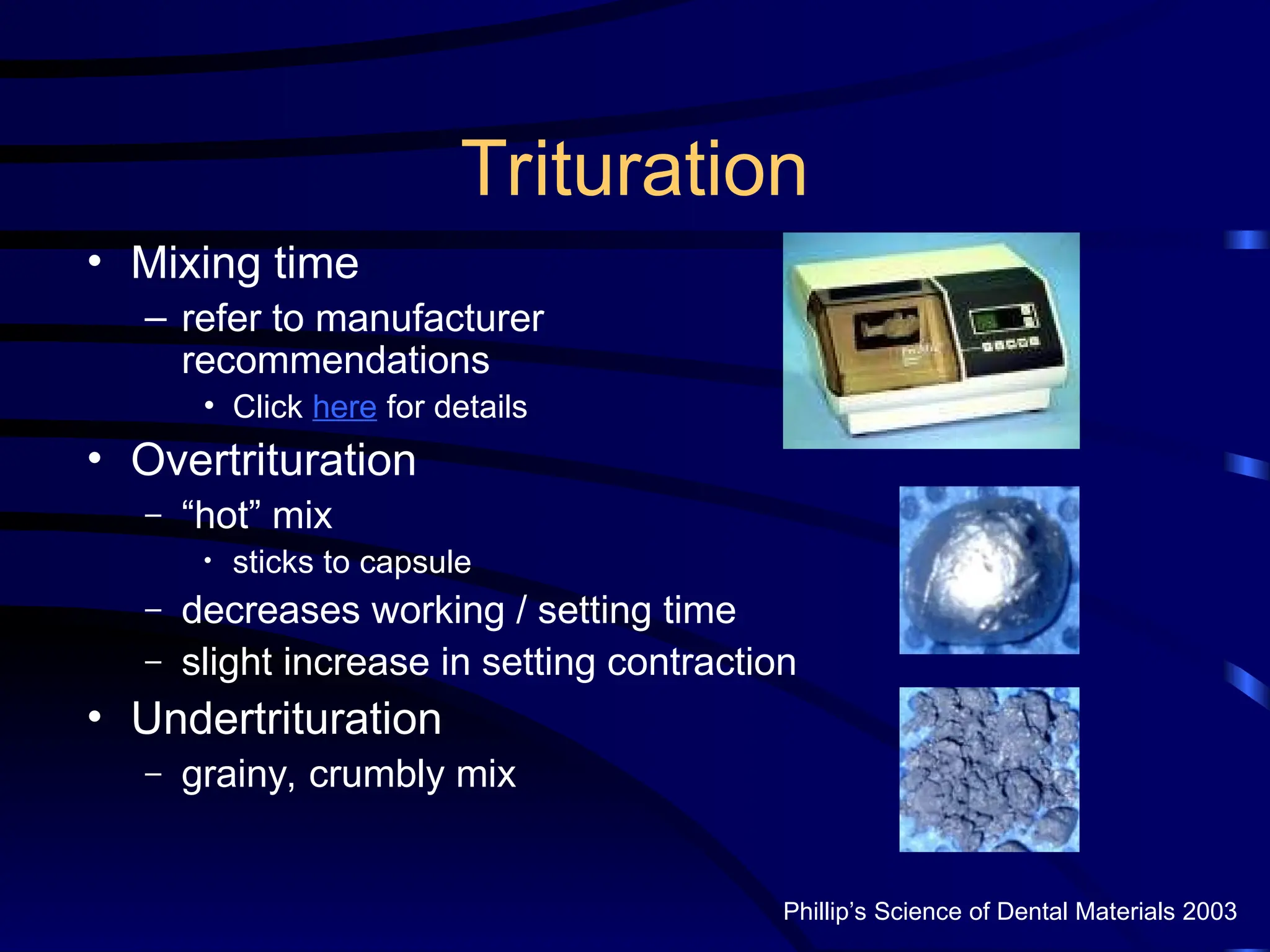
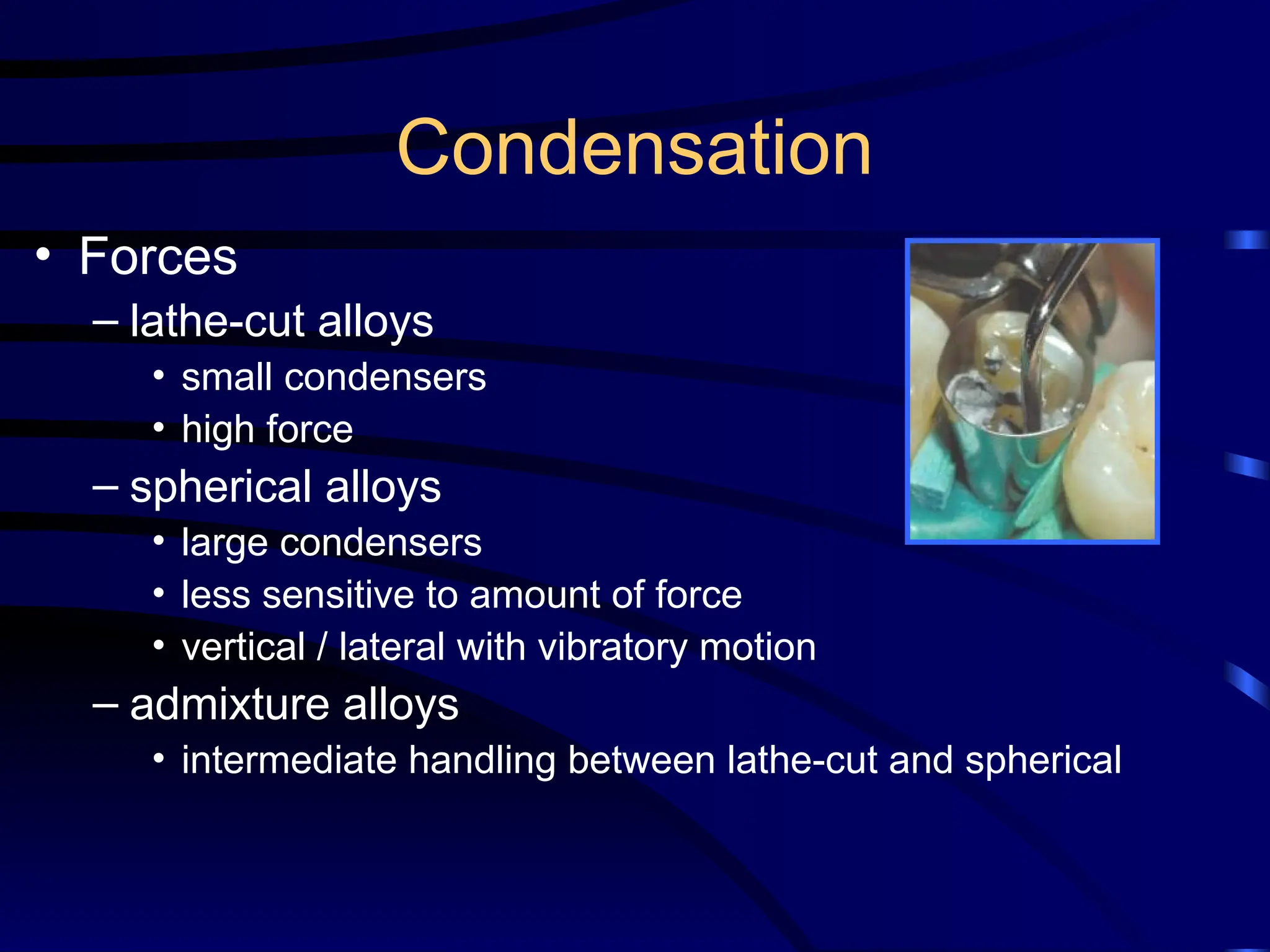
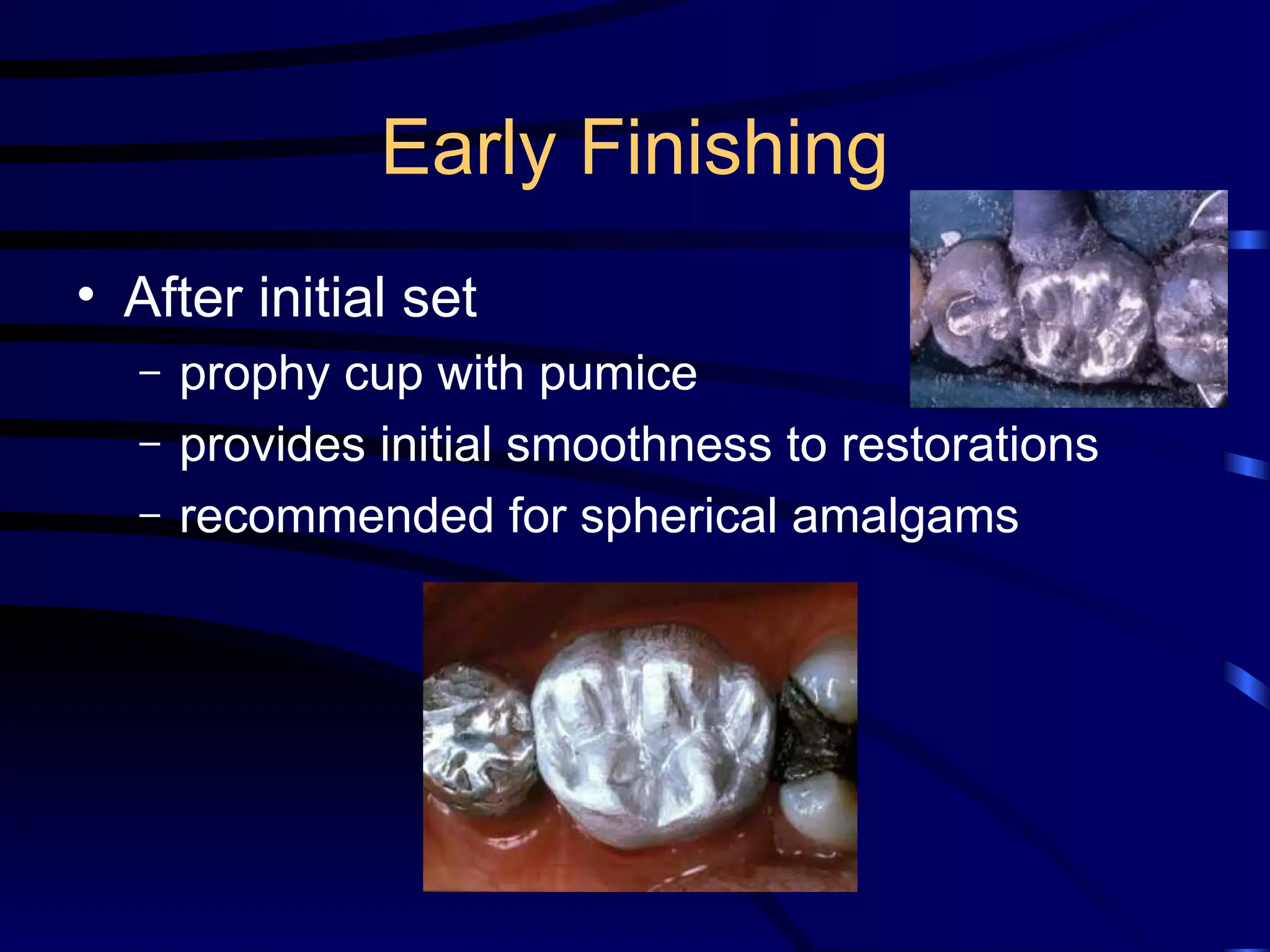
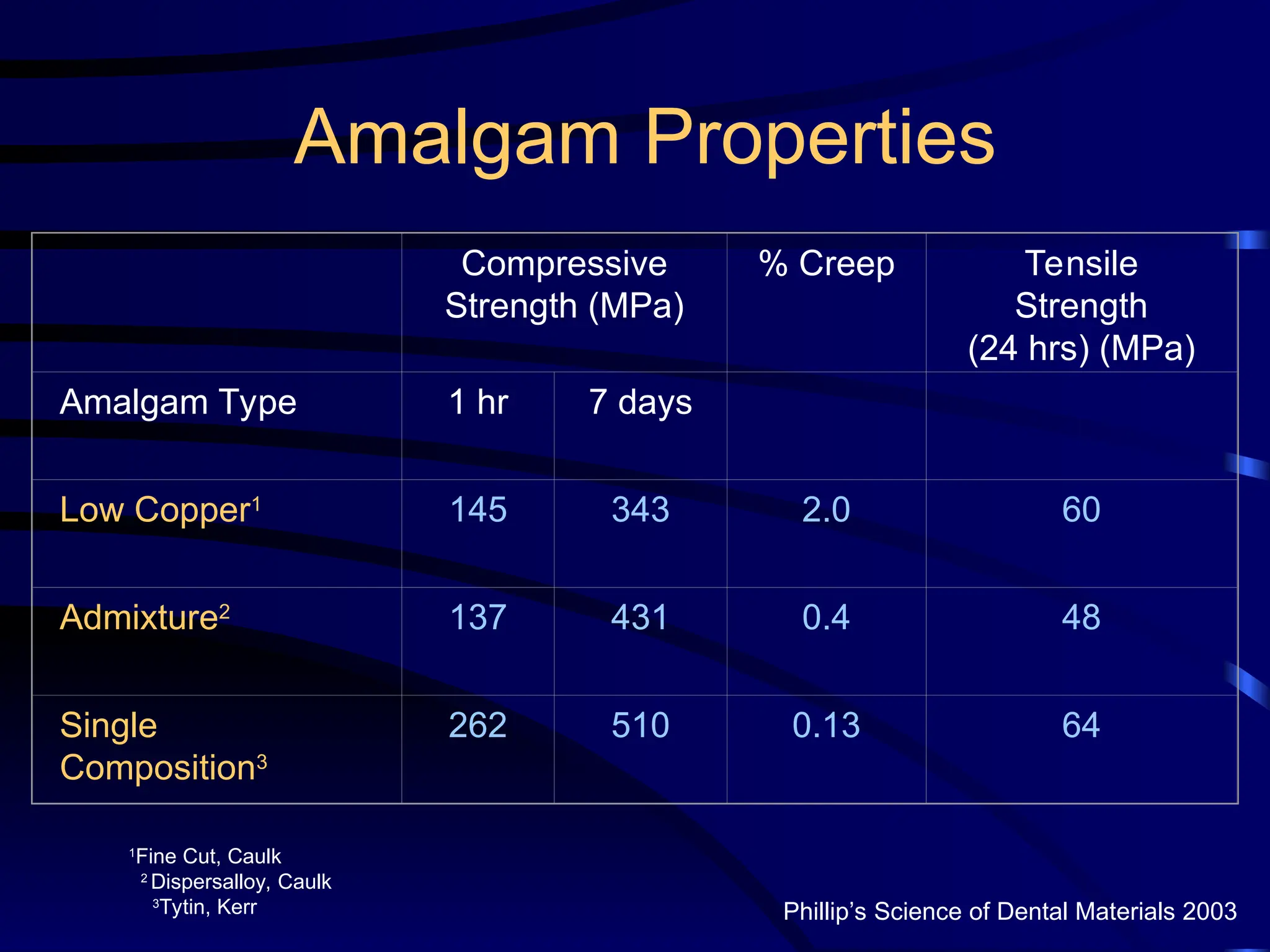
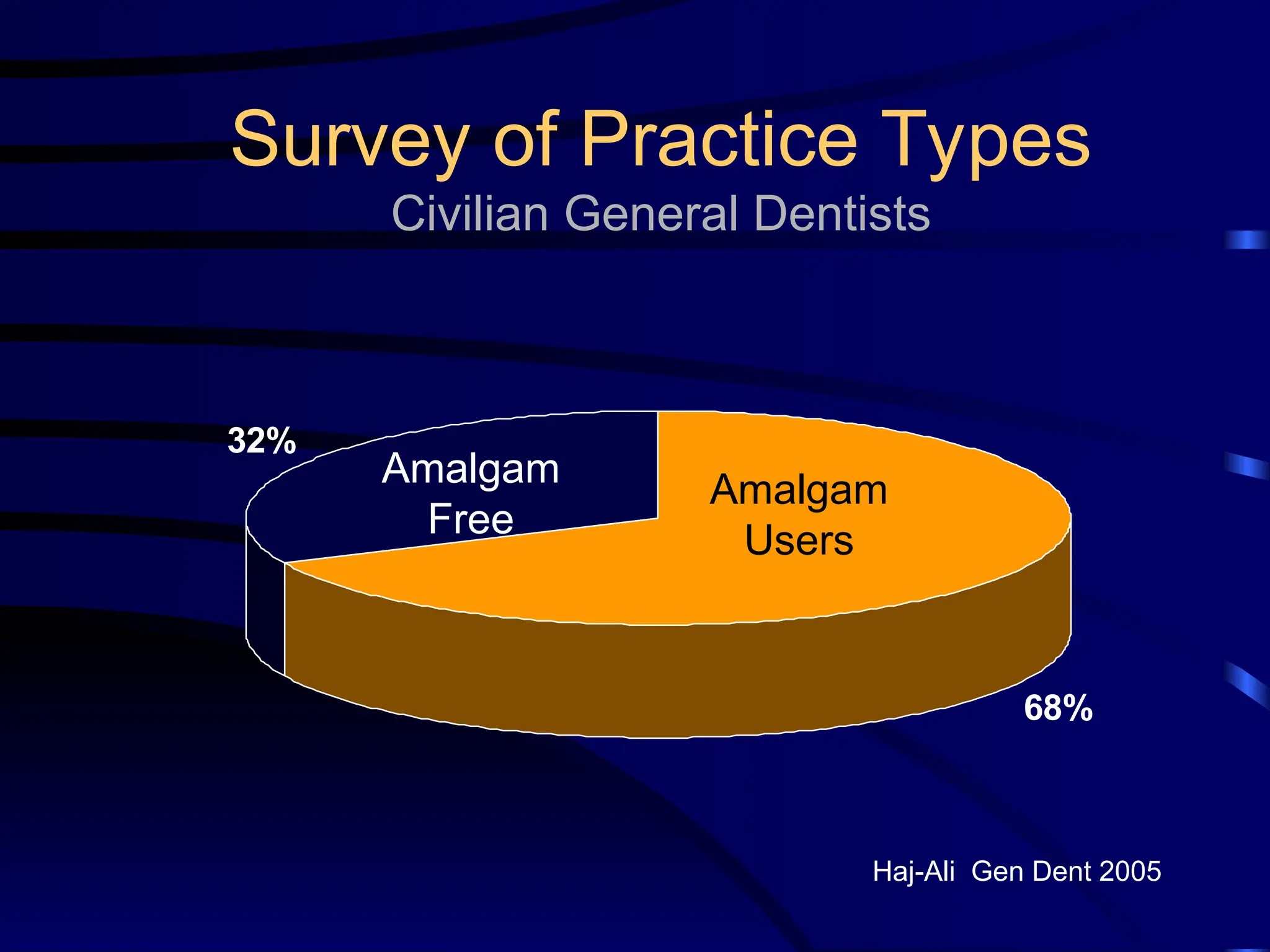
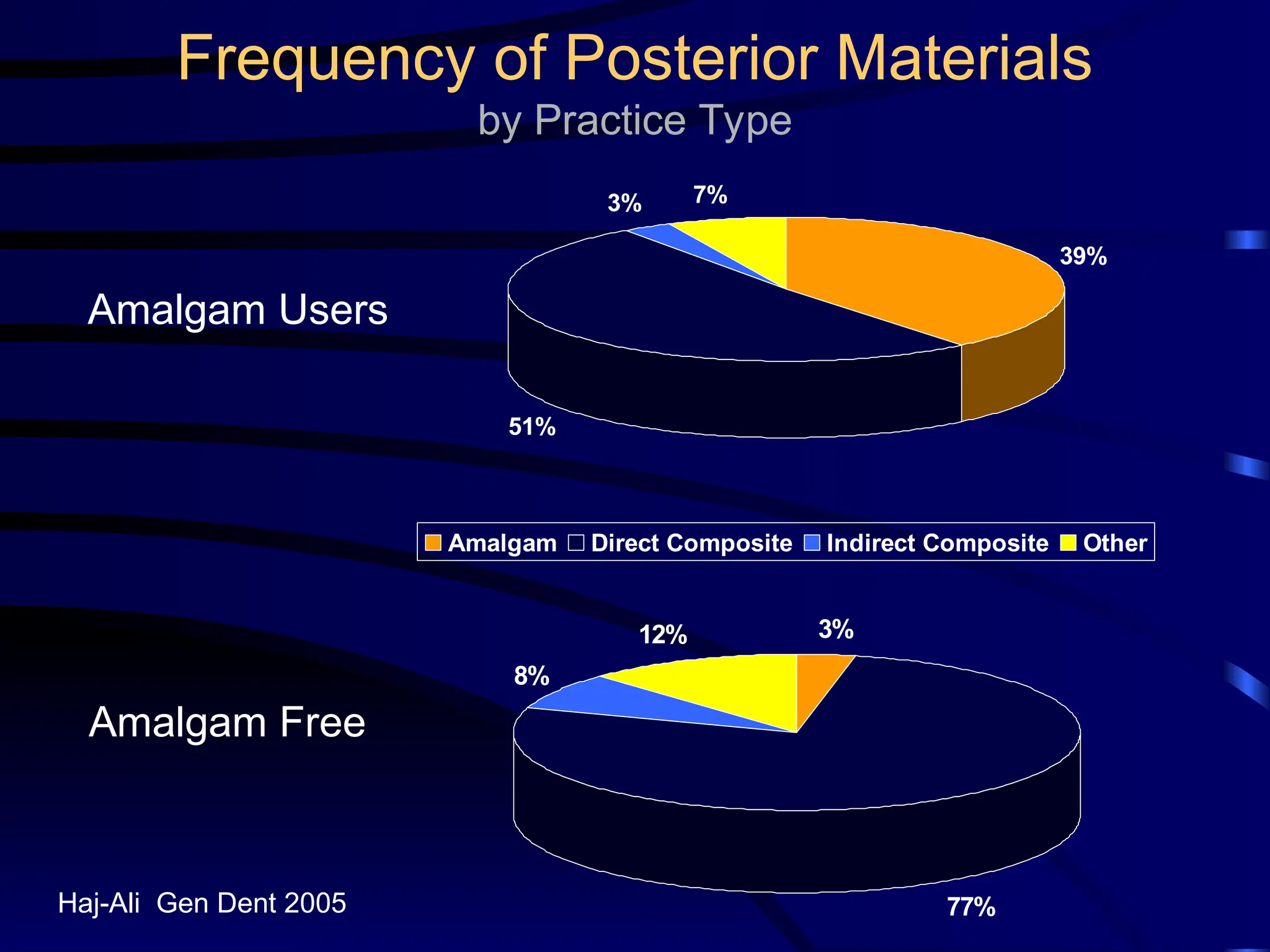
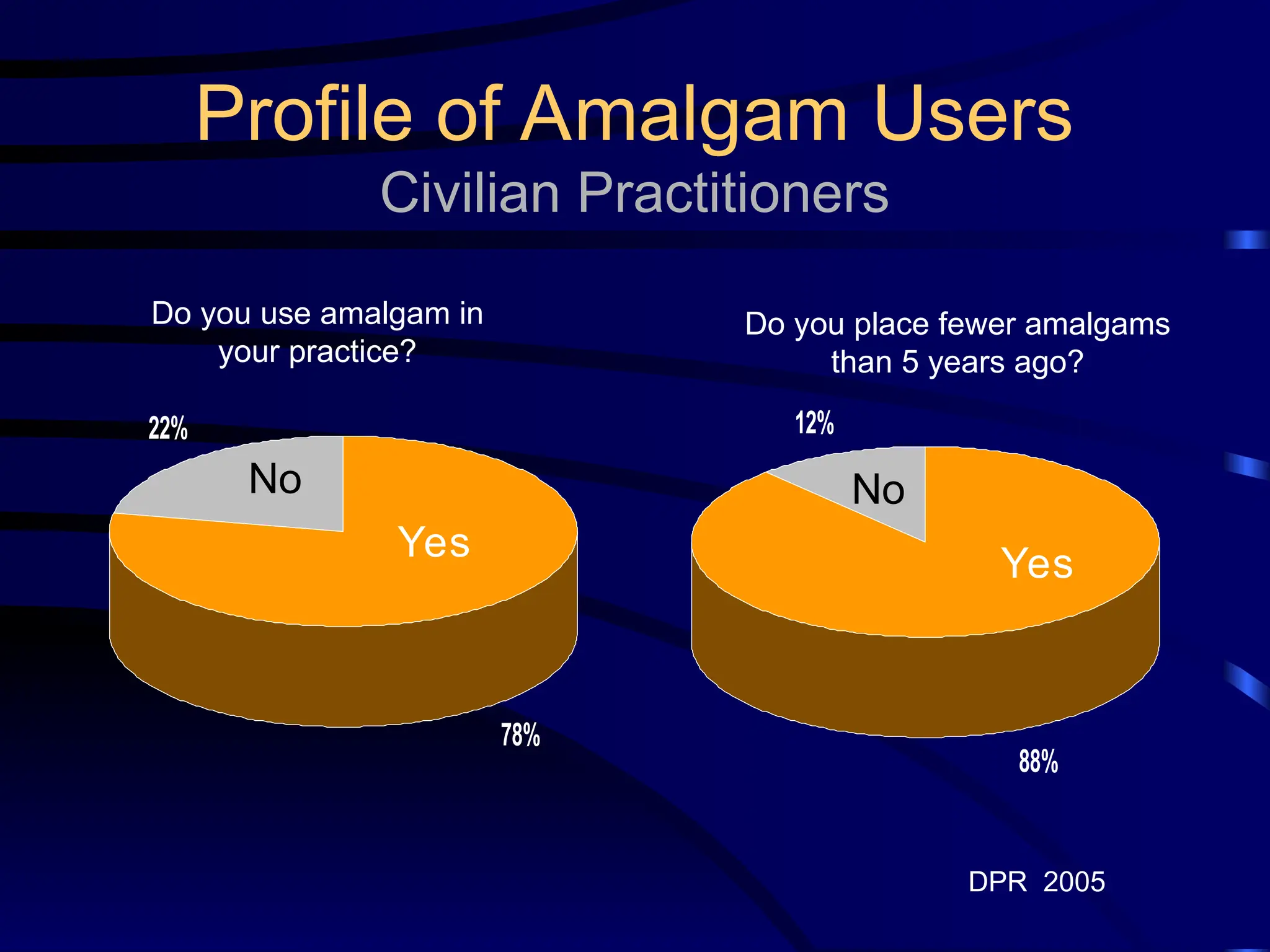
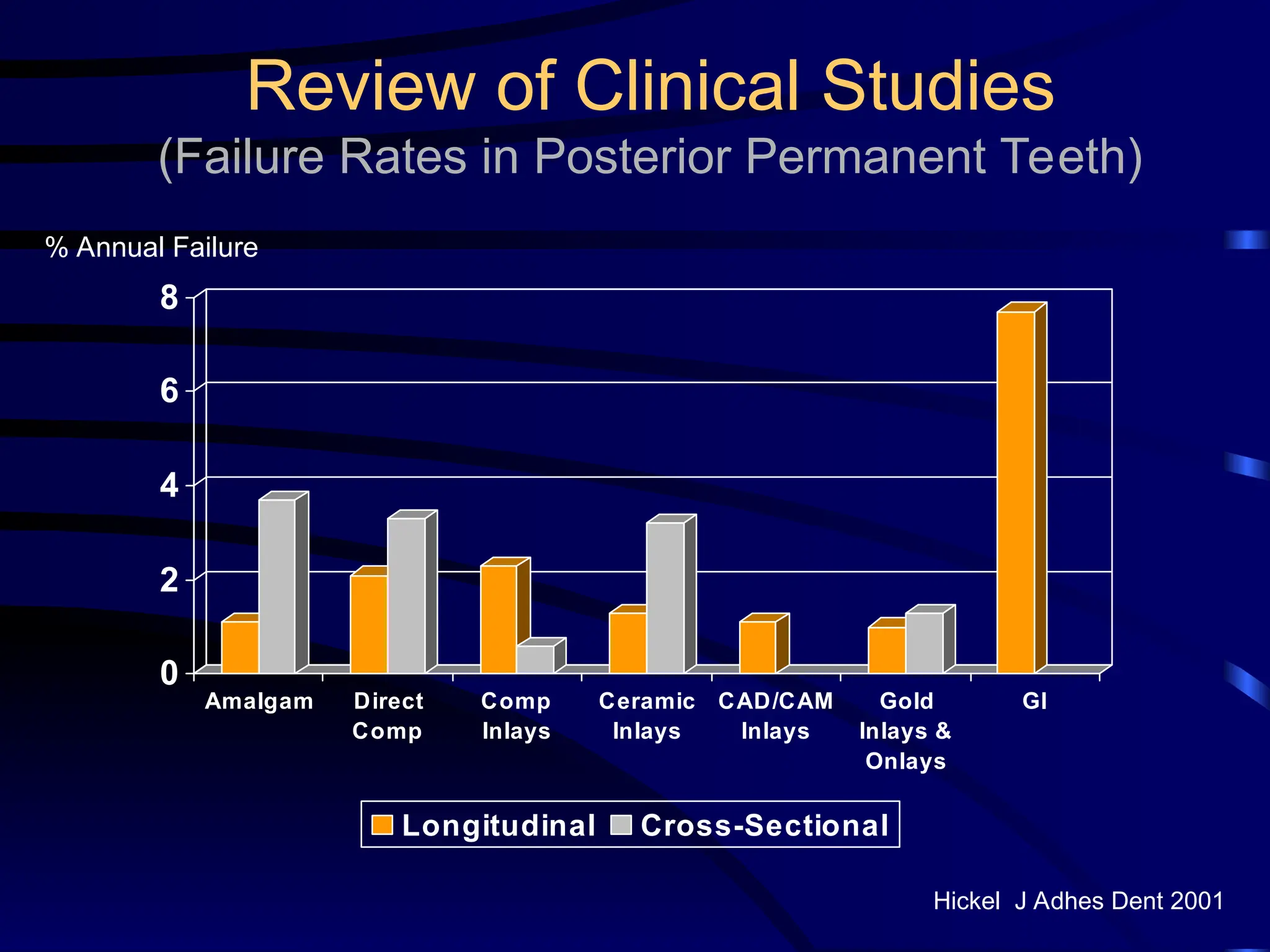
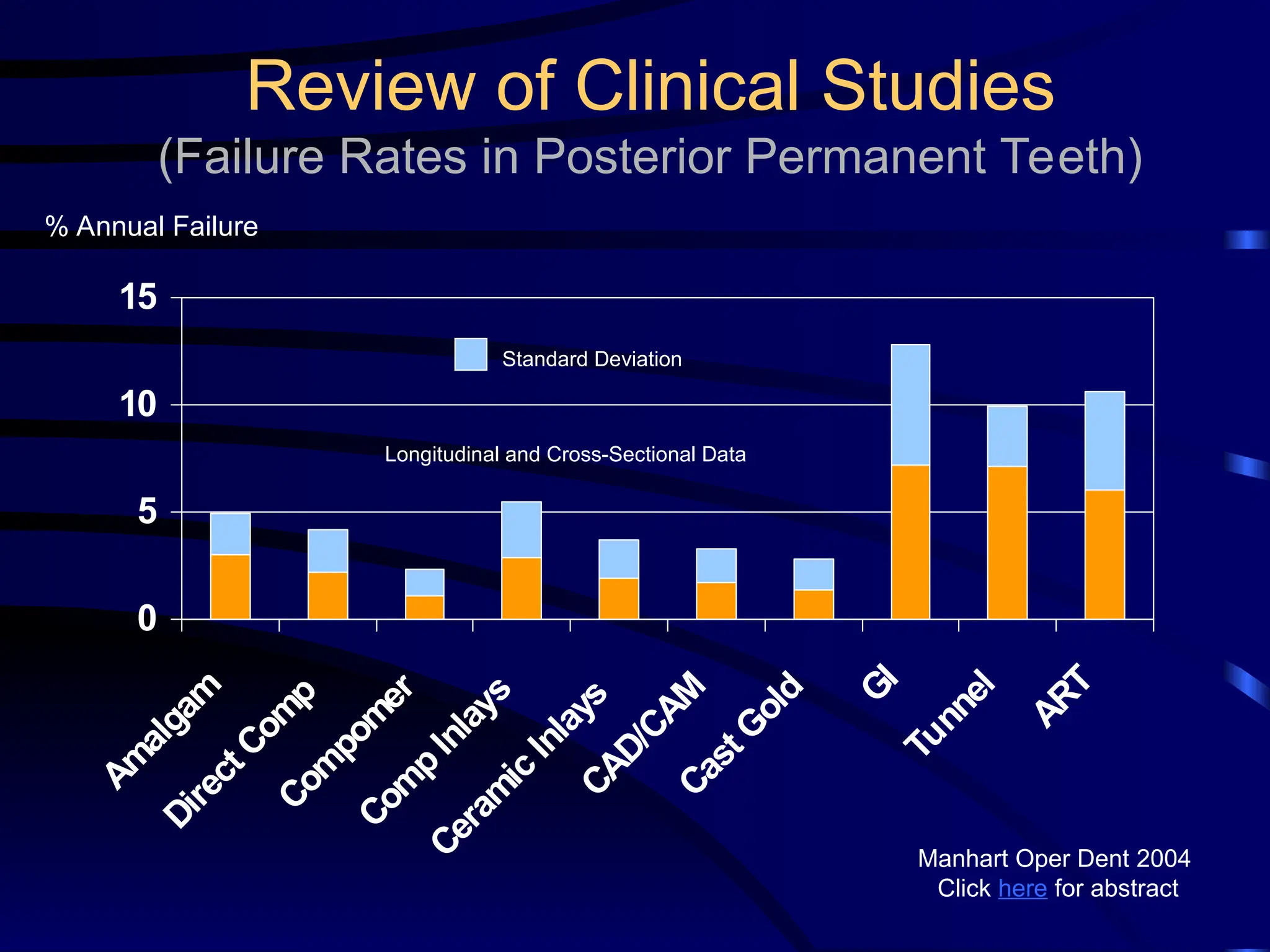
The document provides a comprehensive overview of dental amalgam, including its history, composition, classifications, and manufacturing processes. It discusses the properties and advantages of different types of amalgams, as well as factors that affect their performance, such as corrosion and dimensional changes. Additionally, it addresses the prevailing trends in amalgam use among dental practitioners and clinical performance data.