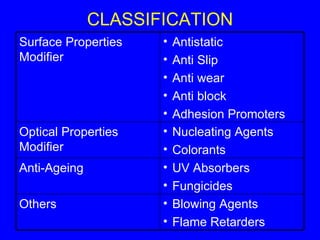
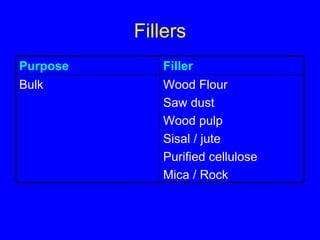
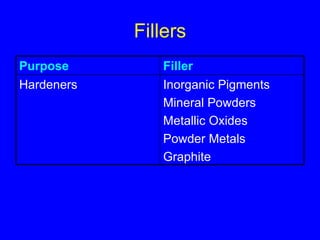
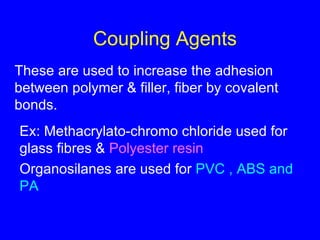
This document discusses various types of additives used in polymer processing and their functions. It describes additives like stabilizers, lubricants, plasticizers, fillers, fibers, coupling agents, antistatic agents, slip agents, anti-block agents, nucleating agents, optical brighteners, colorants, anti-aging additives, impact modifiers, flame retardants, blowing agents, and master batches. It provides examples and explains how each additive type alters polymer properties or facilitates processing to achieve the desired characteristics in final products.