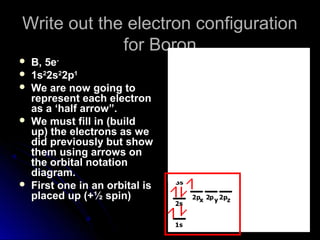
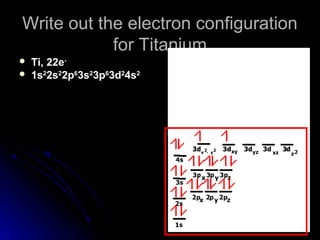
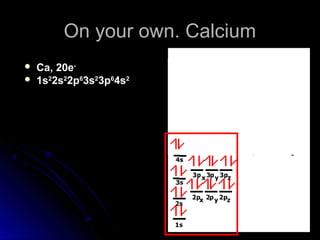
Orbital notation represents the electron configuration of an element by showing the placement of each electron through arrows. It illustrates the Pauli exclusion principle, which states that no two electrons in the same atom can have the same quantum numbers, requiring opposite spin directions. The homework involves writing out electron configurations using orbital notation diagrams for various elements like sodium, boron, and titanium.