The document is a student submission that contains responses to questions about electron behavior. It discusses:
1) Centrifugal force arises from an electron moving in a circular path, and acts outwardly away from the center of rotation. The electron experiences centrifugal force from the middle point of its circular orbit.
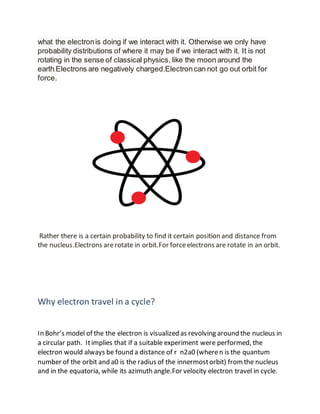
2) Electrons rotate in orbits around the nucleus due to forces. They cannot change energy levels or escape the orbit without external interaction. Their behavior is probabilistic rather than classical circular motion.
3) According to Bohr's model, electrons travel in circular paths or orbits around the nucleus. They cannot escape the cyclic path due to forces acting on them and can only be found at certain distances from the