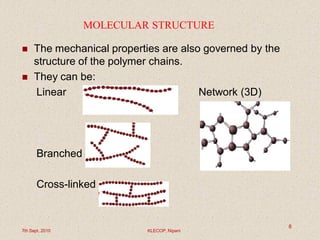
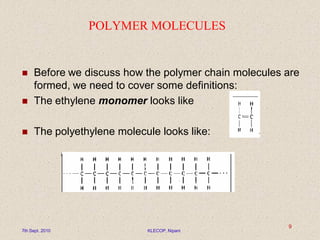
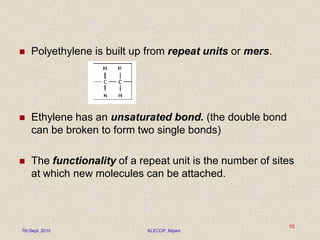
This document discusses polymers and their applications in drug delivery. It begins with an introduction to polymers, including their classification and molecular structure. It then covers general mechanisms of drug release from polymers, including diffusion, degradation and swelling. Applications of polymers in conventional dosage forms and controlled drug delivery are presented. The document also discusses biodegradable polymers and natural polymers. It provides details on the classification, characteristics and selection of polymers for drug delivery.







![Study Questions
Define the following terms:
[Polymer, monomer, elastomer, thermoplastic, sphere, cylinder, annealing,
crystallization, polymerization, deformation, condensation, diffusion, degradation,
binder, Ocusert, Progestasert, synthesis, micro, nano, mega, ionization, degradation,
protonation, erosion, hydrolysis, homogeneous, heterogeneous, etc]
Respond to the following questions:
Give a descriptive account of chemical nature of a polymeric substance and relate to its physical nature
Give a descriptive account of the phases of matter with logical relevance to state of medicines as they
are taken for their respective therapeutical values
What is viscosity and its relation with fluids
What is surface tension and its association with activities of a substance material with surface area
Describe some key phase changes of materials substance when exposed to some environmental
conditions of change
How is a chemical change different from a physical change](https://image.slidesharecdn.com/13-polymerscience-200630105549/85/13-polymer-science-20-320.jpg)



































![Study Questions
Define the following terms:
[Polymer, monomer, elastomer, thermoplastic, sphere, cylinder, annealing, crystallization,
polymerization, deformation, condensation, diffusion, degradation, binder, Ocusert,
Progestasert, synthesis, micro, nano, mega, ionization, degradation, protonation, erosion,
hydrolysis, homogeneous, heterogeneous, etc]
Respond to the following questions:
Give a descriptive account of chemical nature of a polymeric substance
Give a descriptive account of classification of polymeric material substance with examples
State and explain with examples the characteristics of ideal polymeric material substances
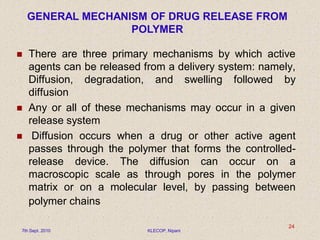
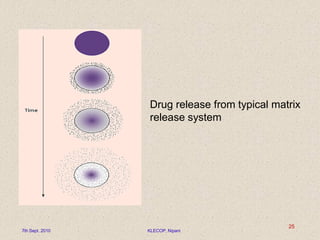
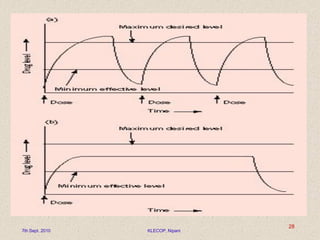
Give a descriptive account of general mechanisms polymeric material substance in particle release processes
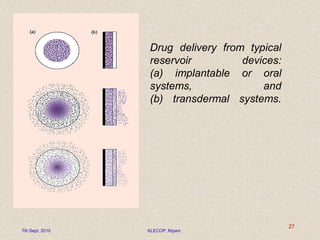
Describe the pharmaceutical fields of applications using polymeric materials
Give a detailed material nature of biodegradable polymeric substances
List and elaborate on the advantages and disadvantages of biodegradable polymeric material use
pharmaceutically
Illustrate the use of biodegradable polymers for the present-time pharmaceutical care service provision
Describe the erosive mechanisms of polymer material from another material substance
How is a chemical nature of polymeric substance different from its physical nature](https://image.slidesharecdn.com/13-polymerscience-200630105549/85/13-polymer-science-66-320.jpg)
