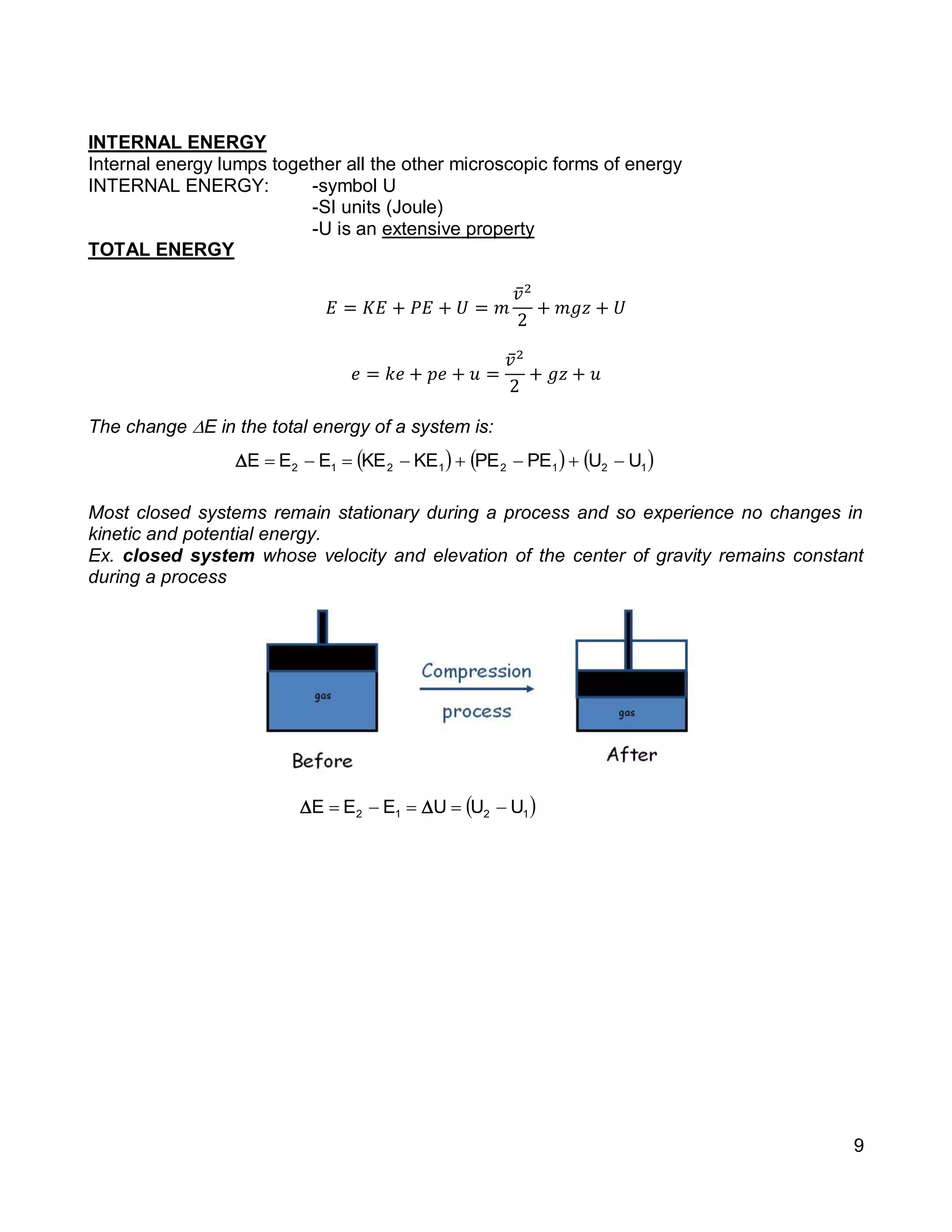
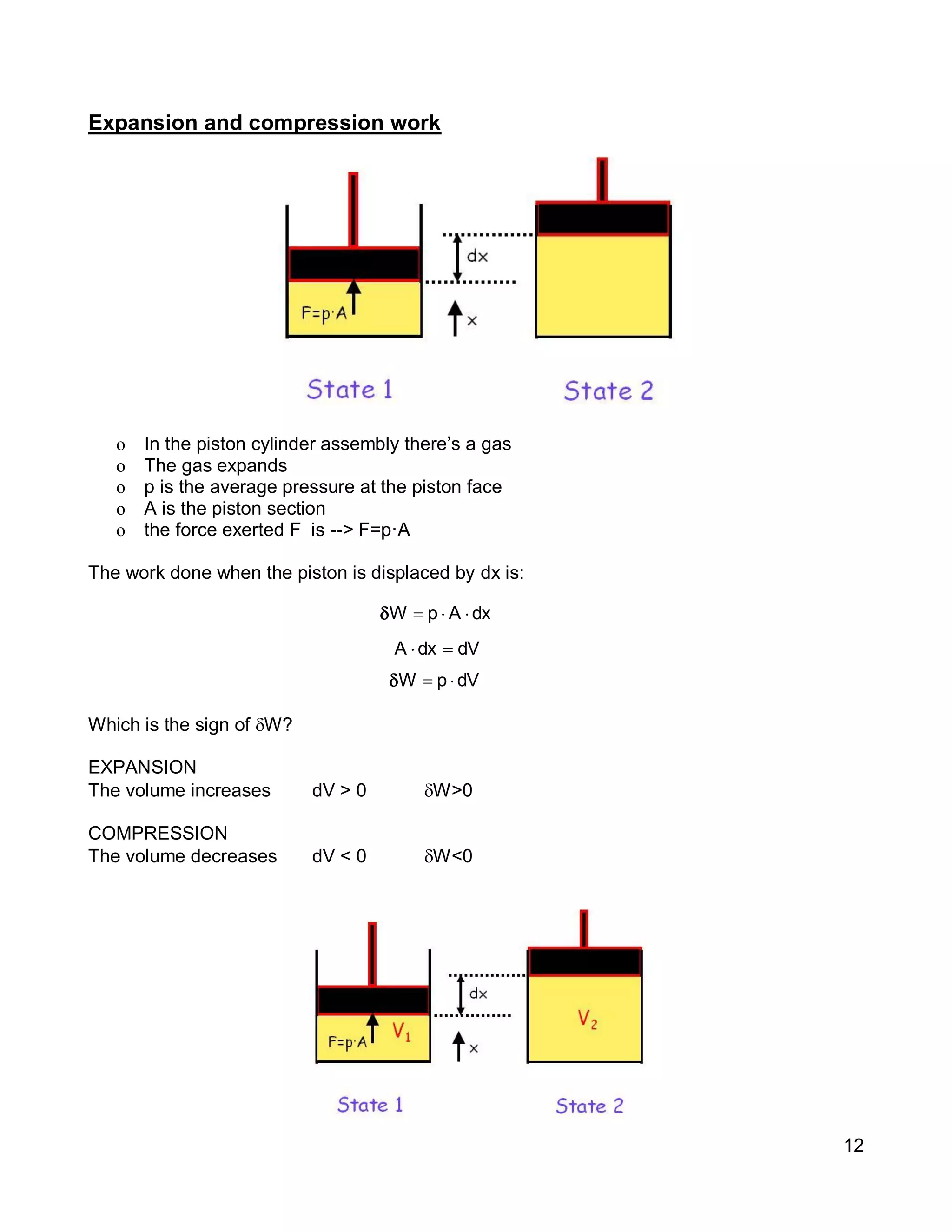
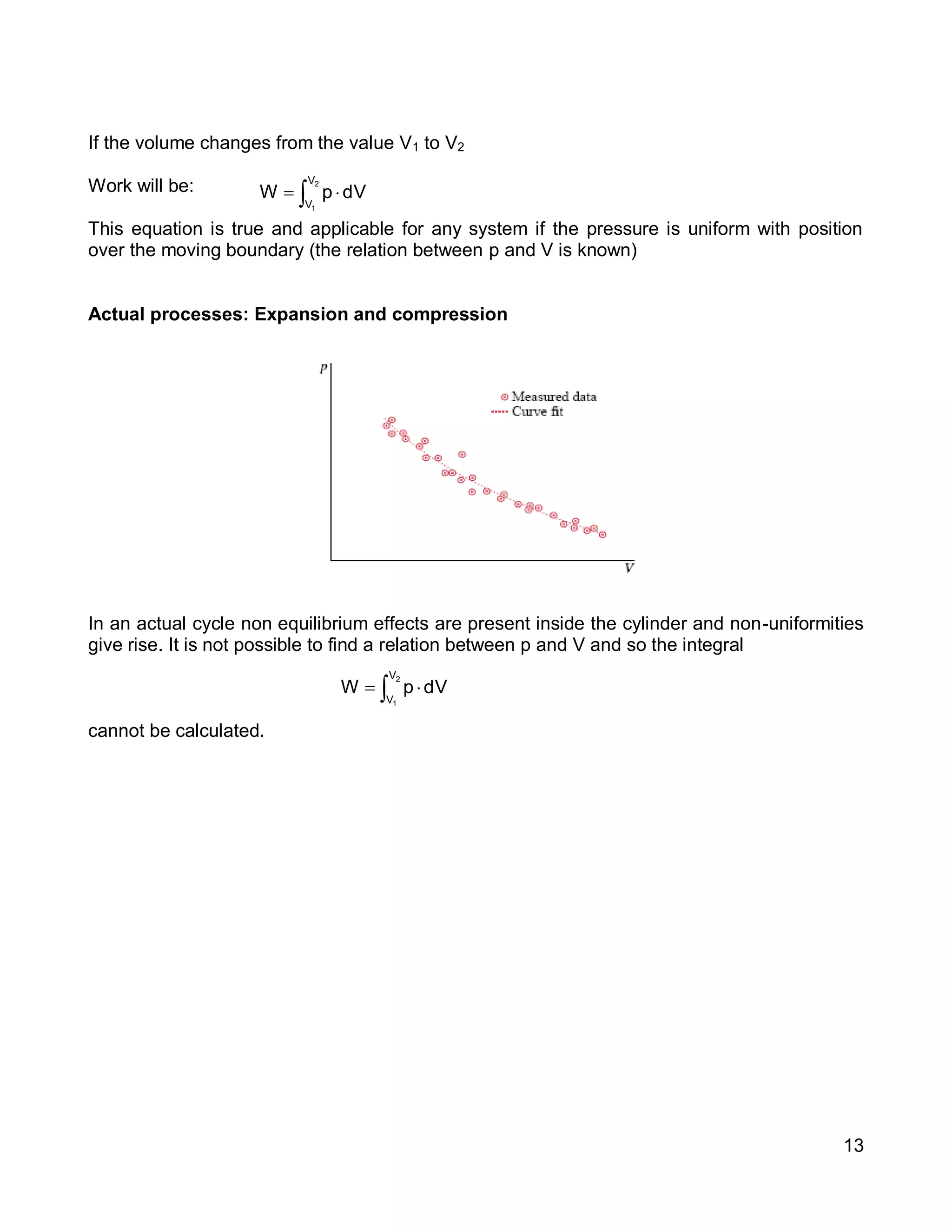
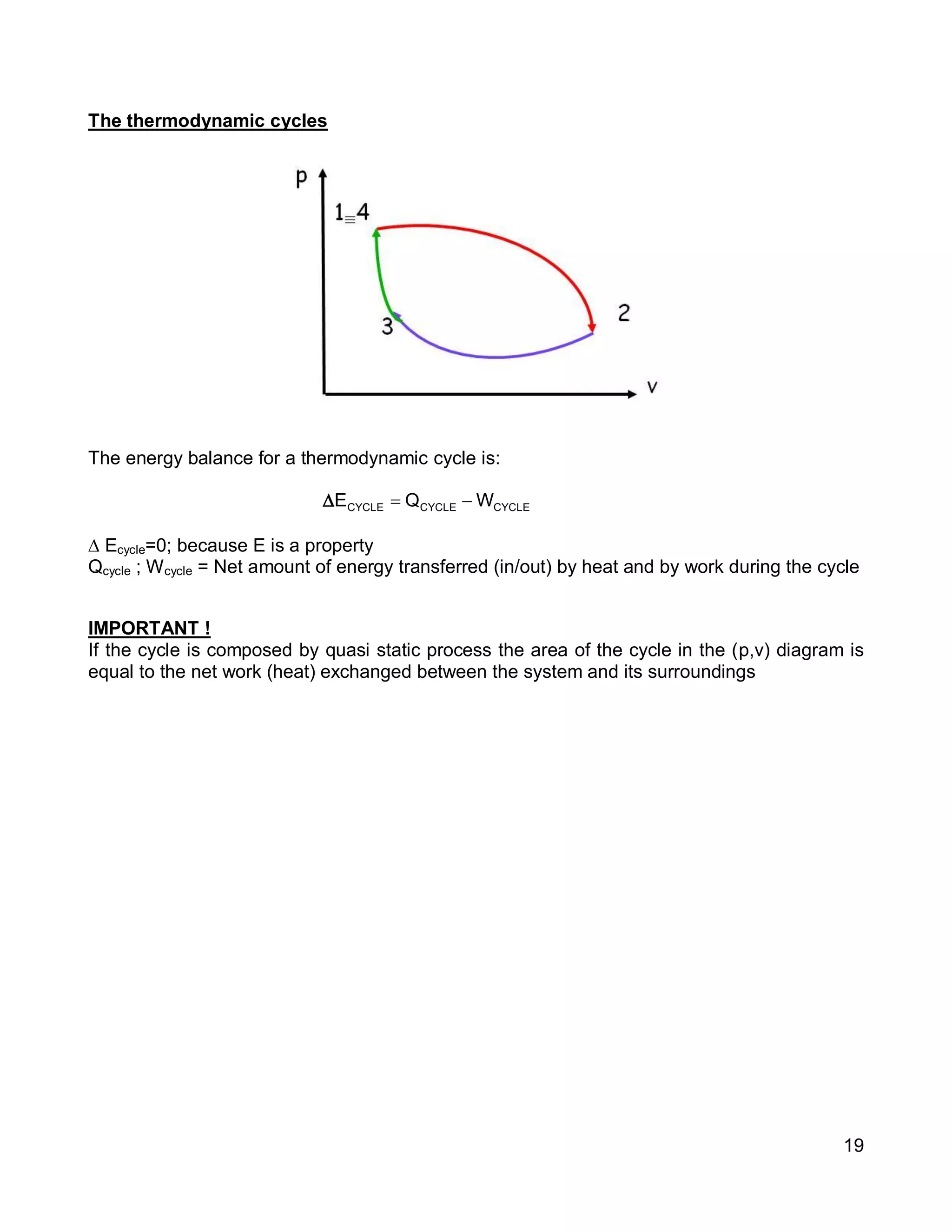
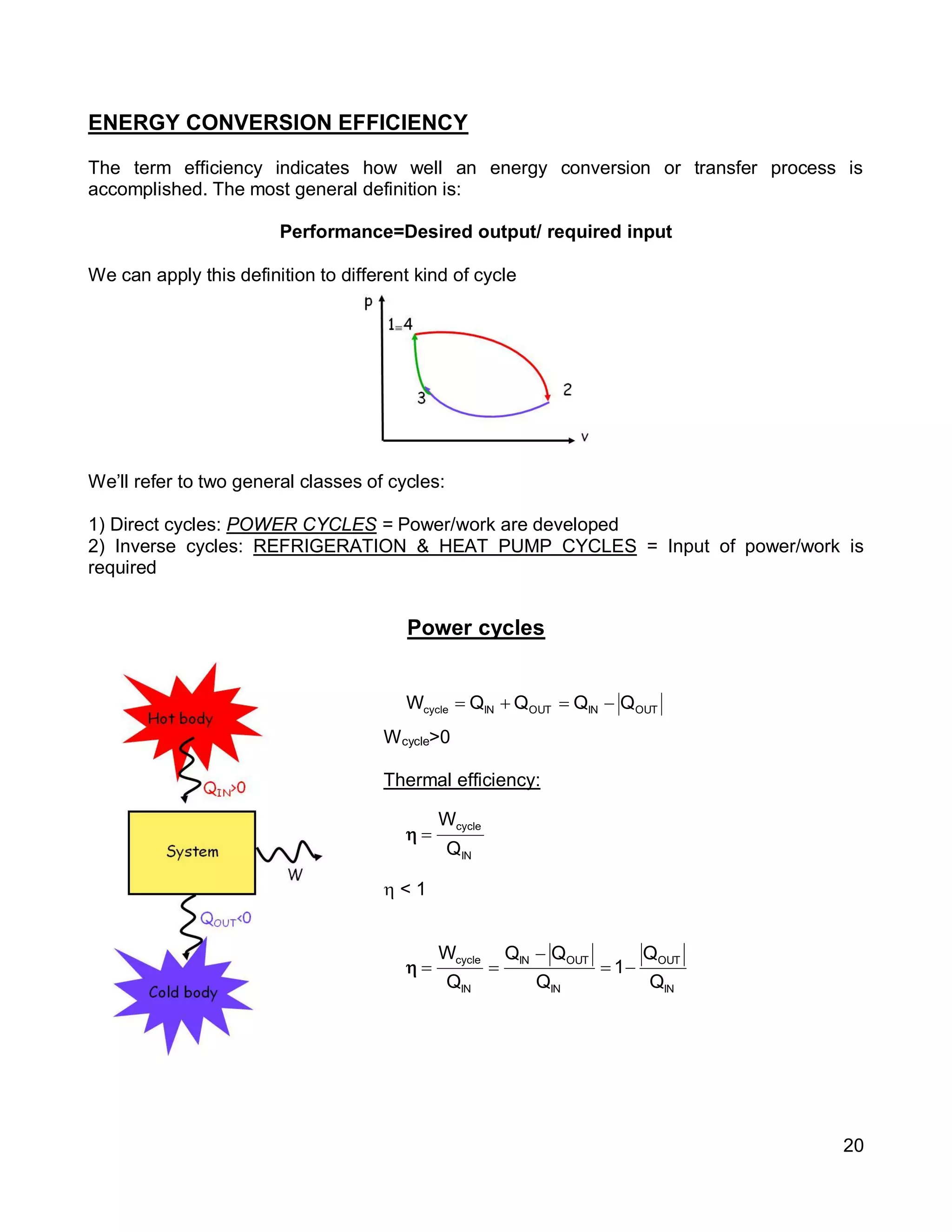
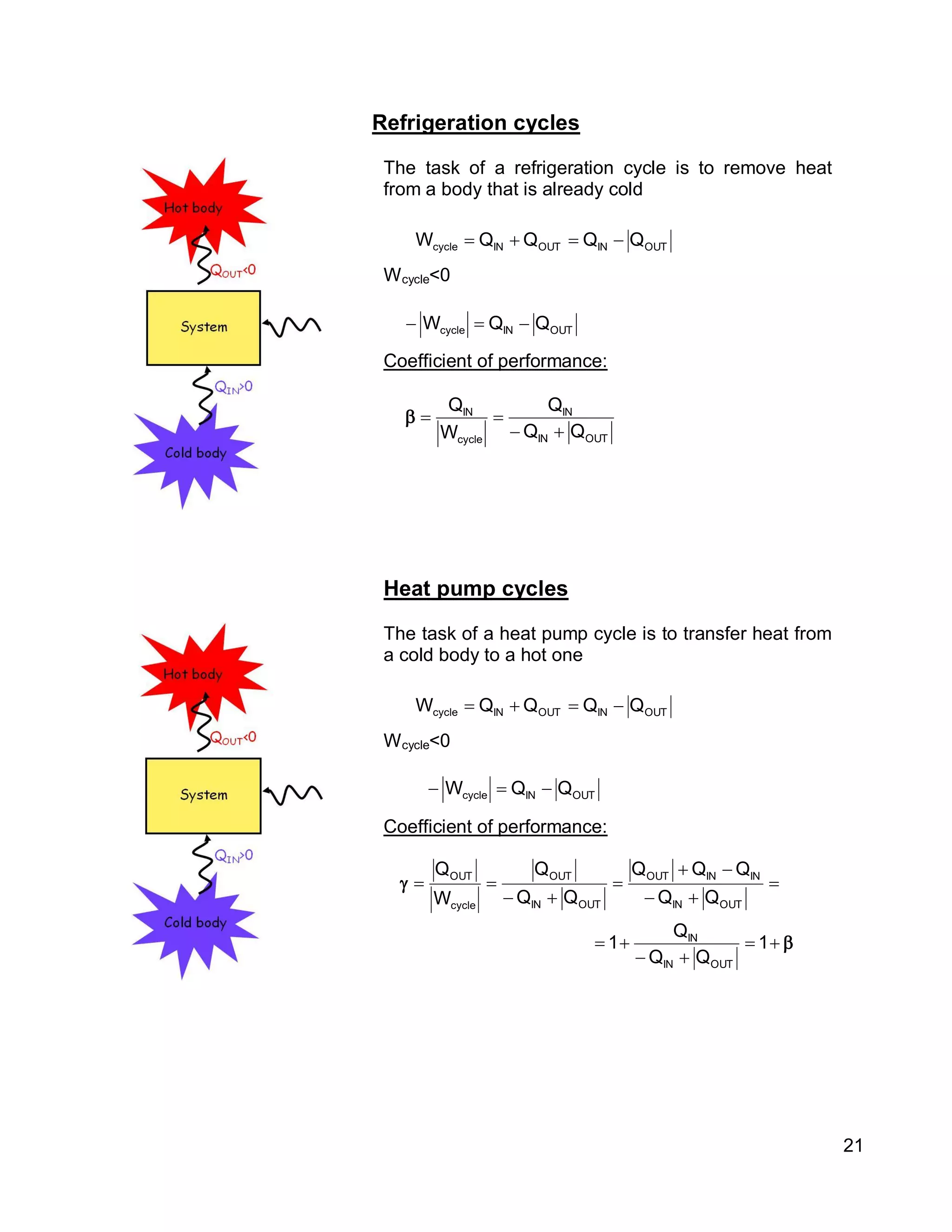
1. The document provides an introduction to thermal systems and the first law of thermodynamics for closed systems.
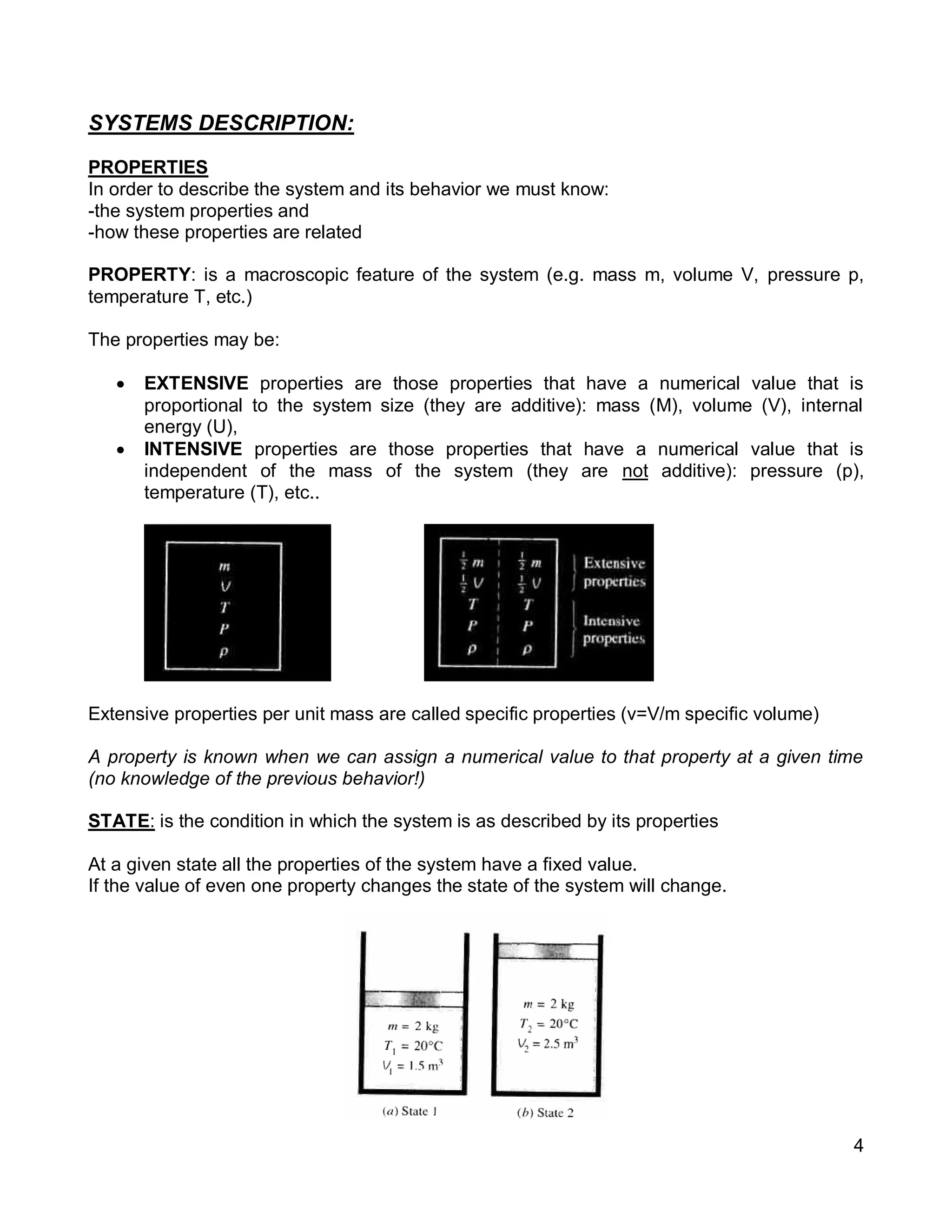
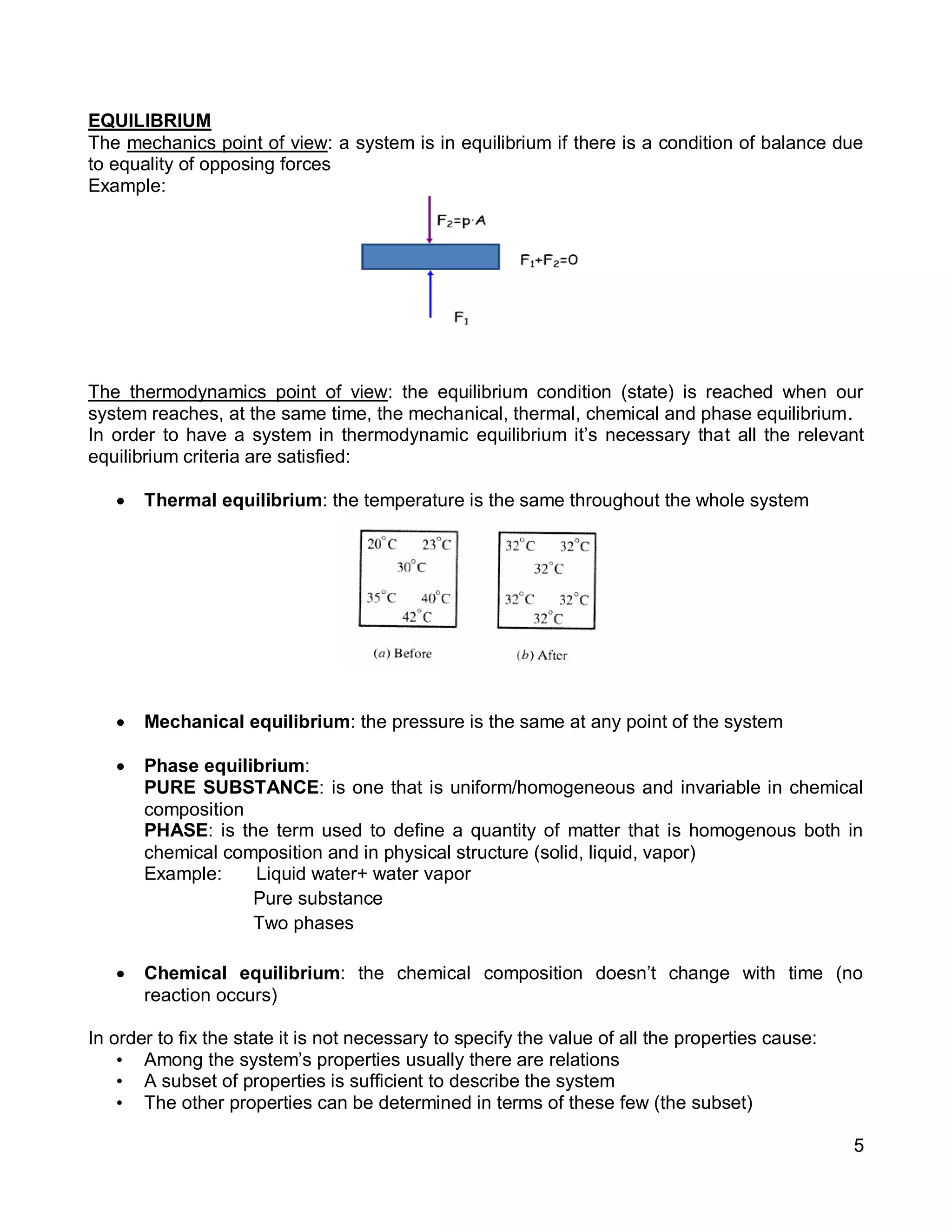
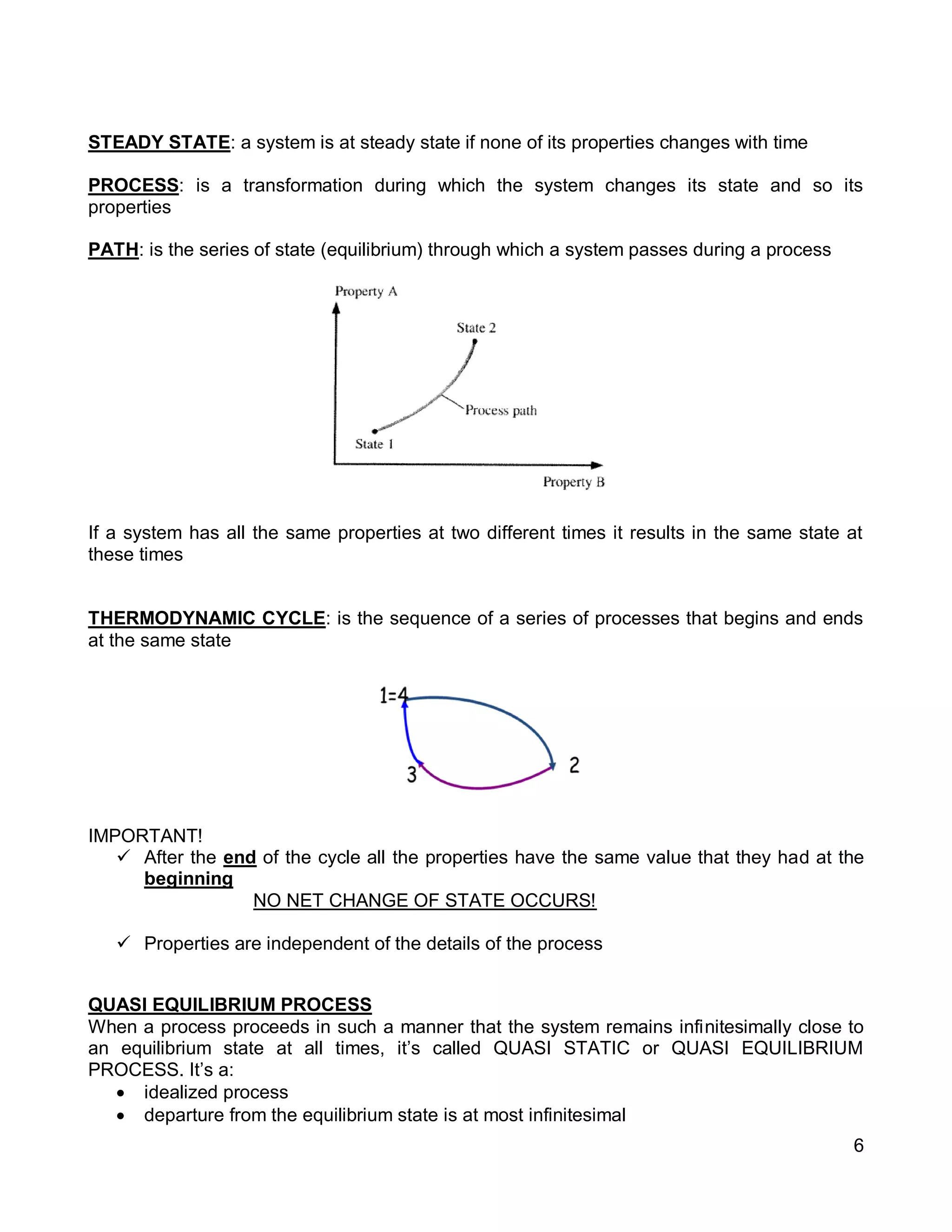
2. It defines key concepts such as systems, surroundings, properties, state, equilibrium, steady state, processes, cycles, and forms of energy including heat and work.
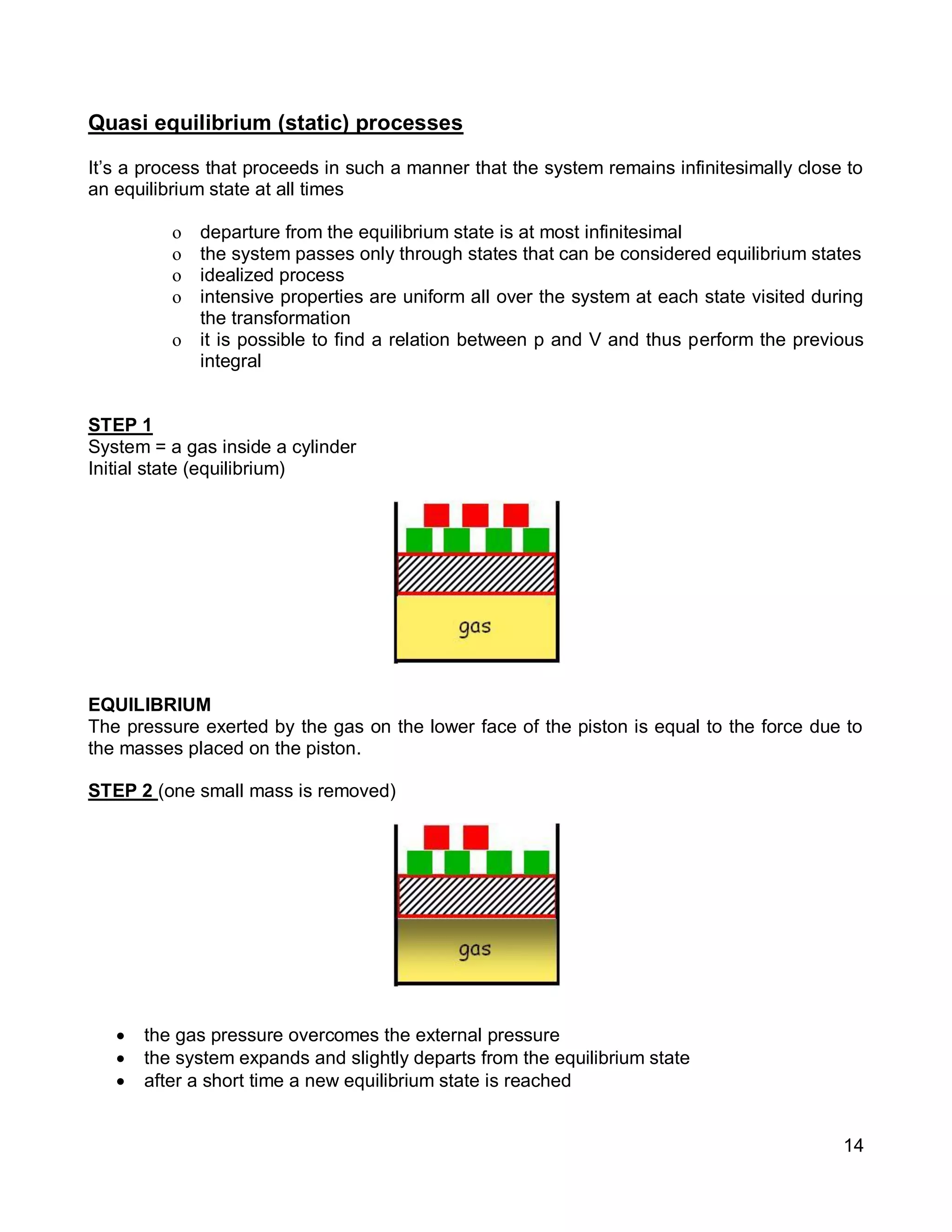
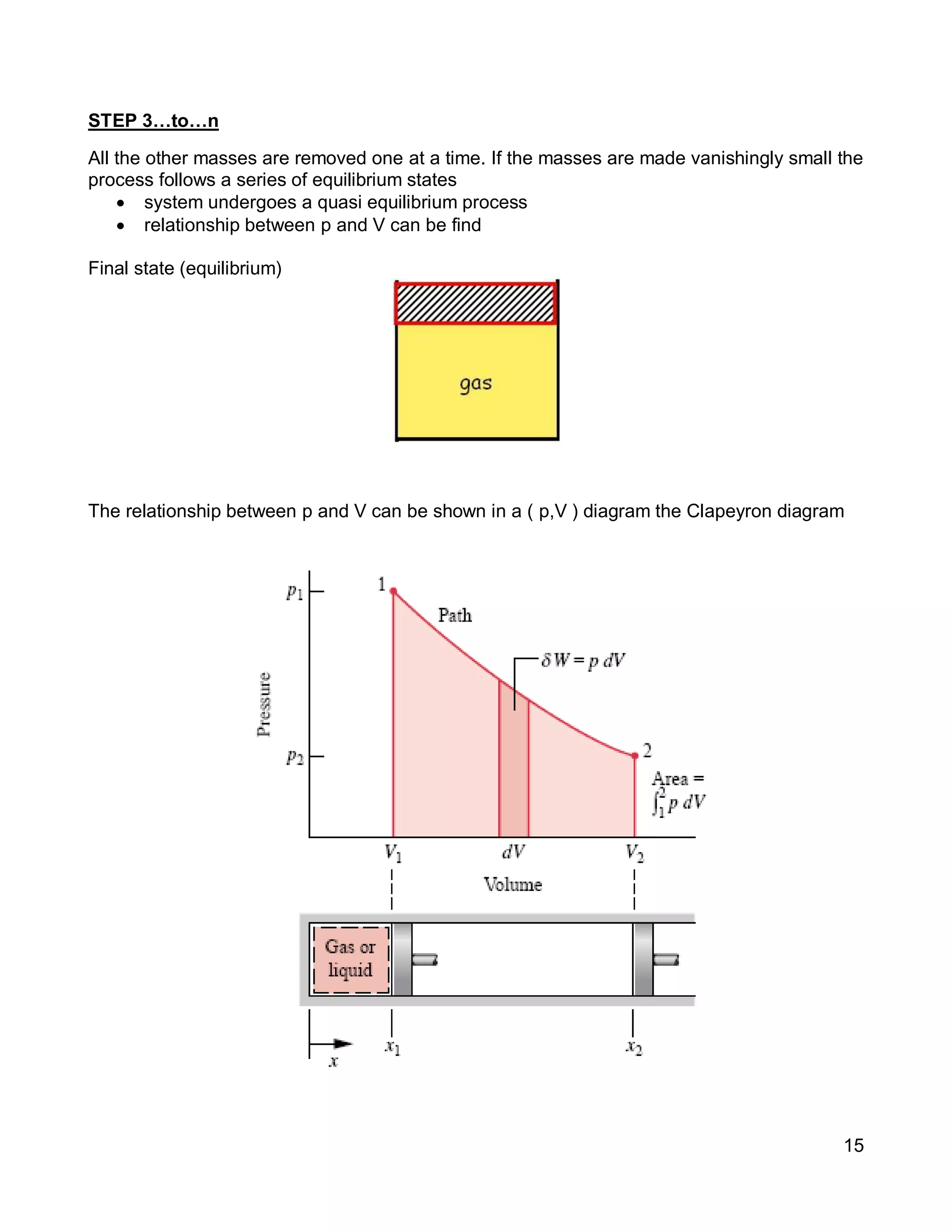
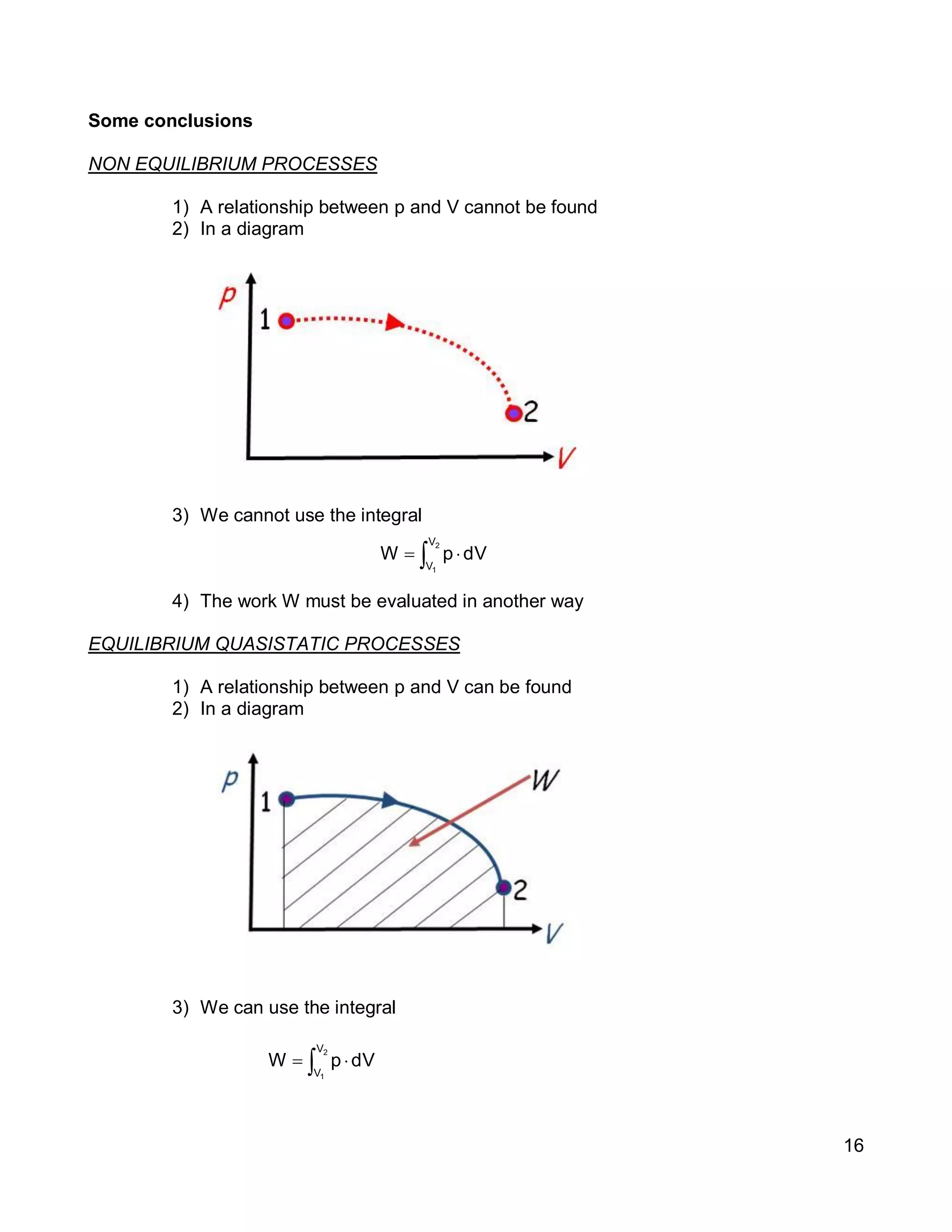
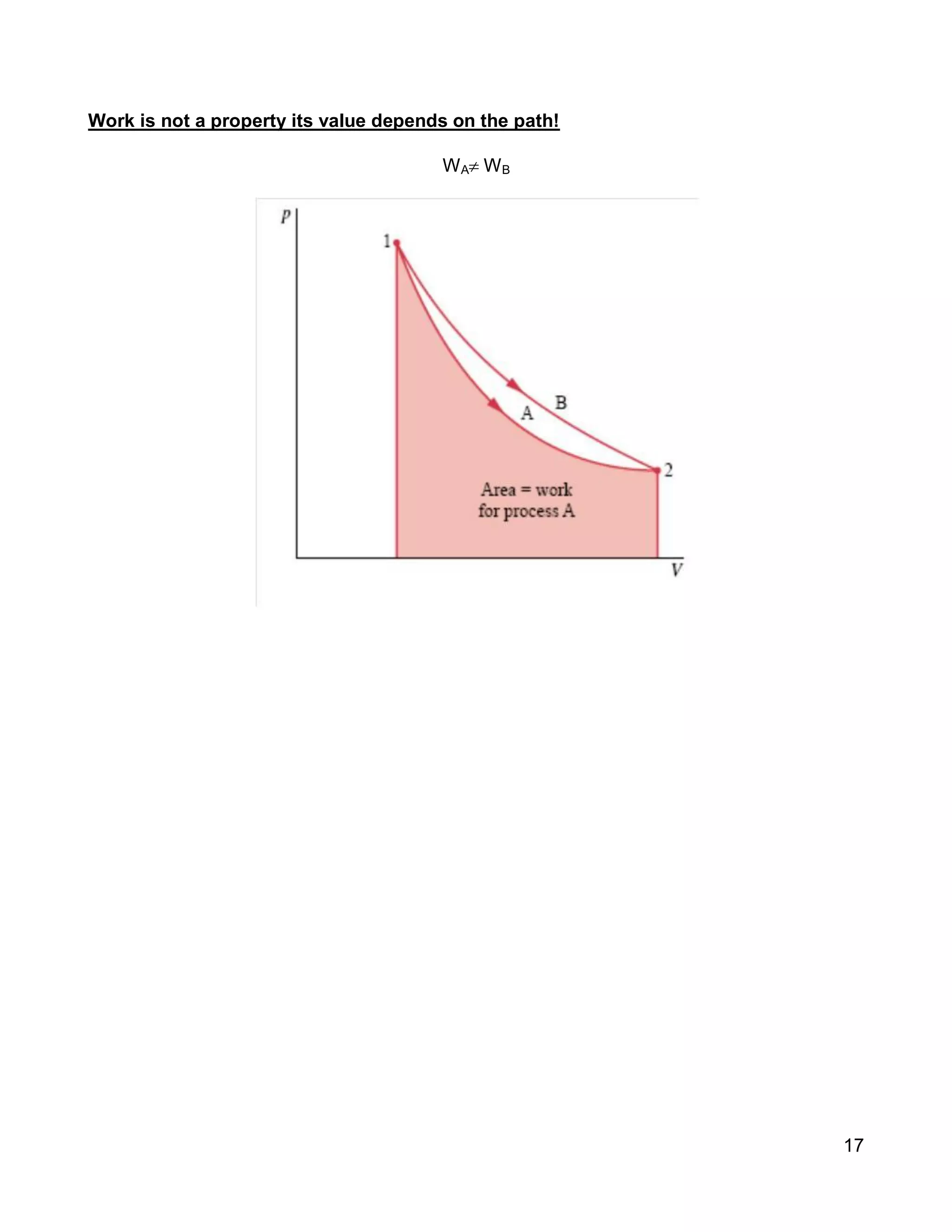
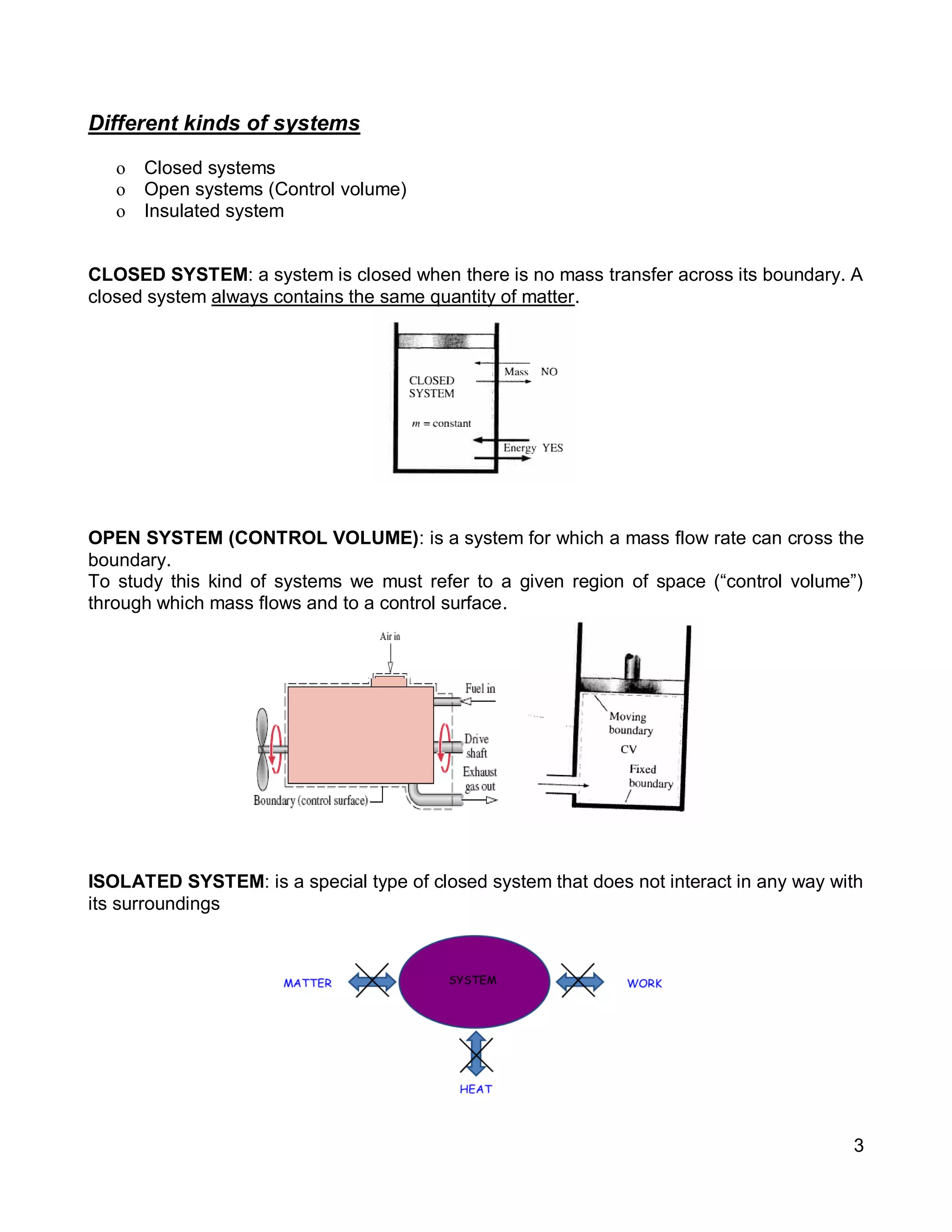
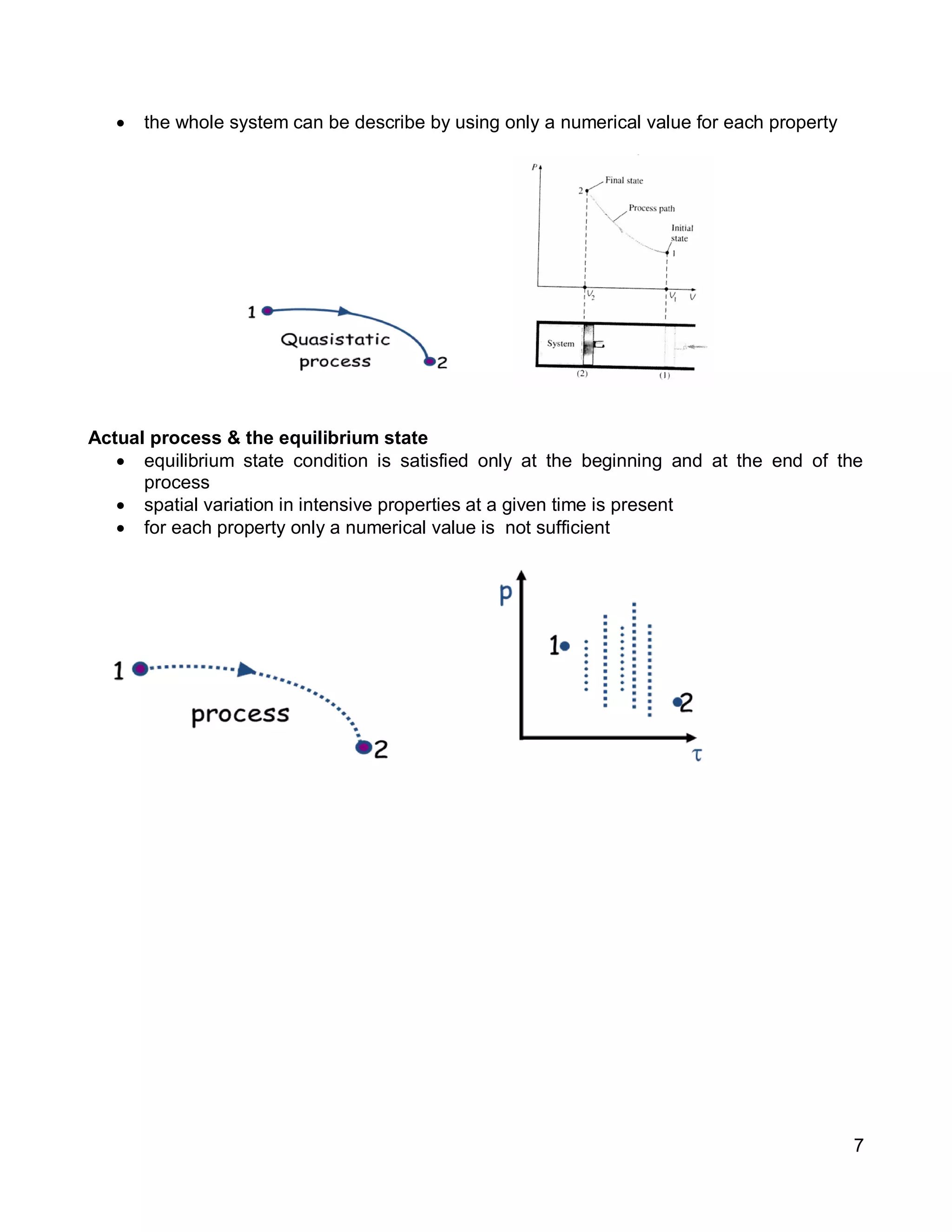
3. Thermal systems can be closed, open, or isolated and undergo processes that may or may not be quasi-equilibrium. The first law relates changes in internal energy of closed systems to heat and work.

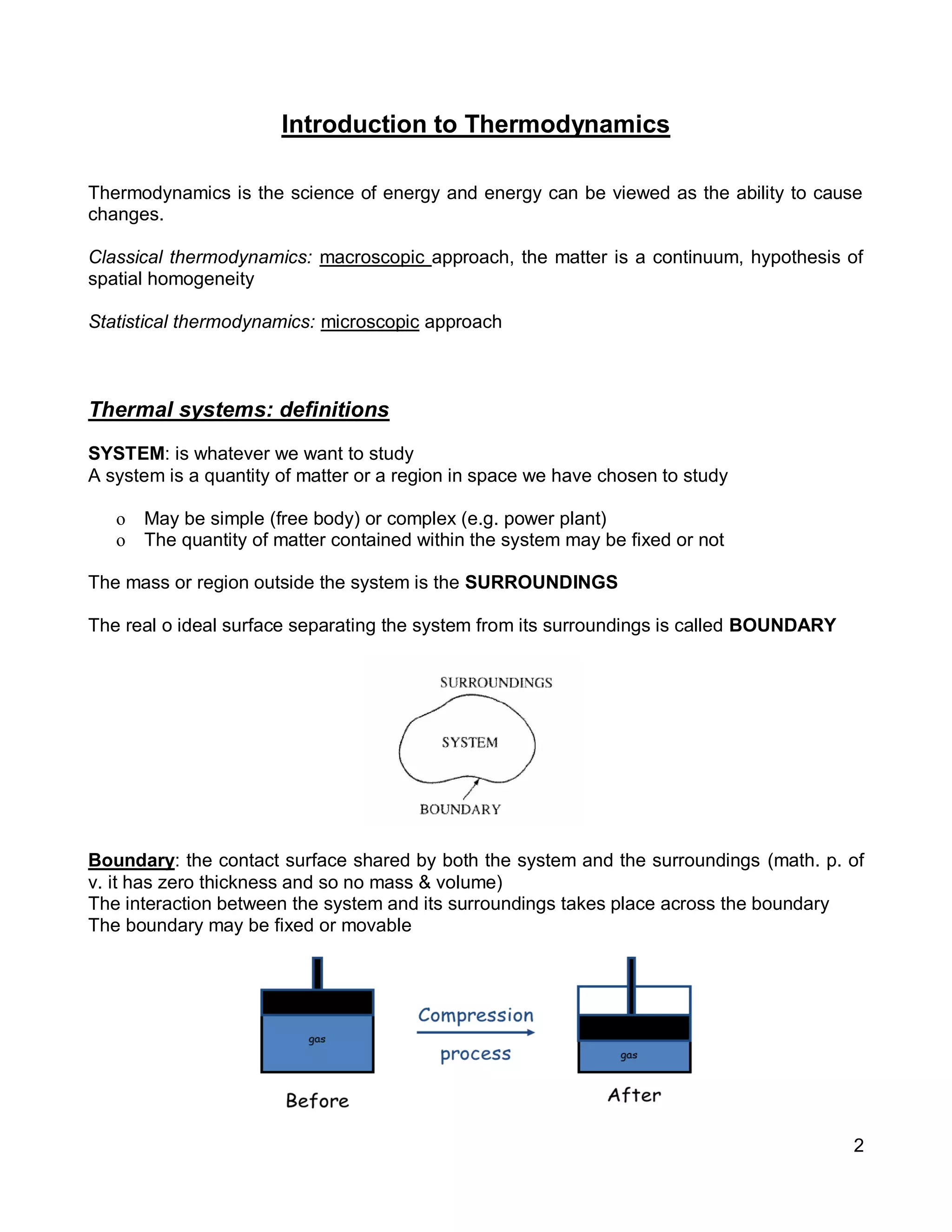
![8
FORMS OF ENERGY:
Thermal systems involve energy:
Storage
Transfer
Conversion
STORAGE: (within the matter that constitutes the system): kinetic energy, gravitational
potential energy, etc.
TRANSFER: (between system and its surroundings): work, heat transfer, flow of streams of
matter
CONVERSION: (from a form to another): e.g. energy associated to combustion process
Energy exists in different for such as: thermal, mechanical, kinetic, potential, magnetic,
chemical, nuclear, etc. Their sum constitutes the total energy E of the system (J) on a unit
mass basis e (J/kg)
We are not interested in the absolute value of the total energy (E=0 in an assigned reference
point) but we’re interested in its change.
In thermodynamic we’ll subdivide the forms of energy that make up the total energy in two
groups:
Macroscopic forms: the ones the system has in respect to an outside reference frame
(surroundings)
Microscopic forms: the ones related to the molecular activity and they’re independent
of any outside reference
KINETIC ENERGY
Body of mass m moving from point 1(𝑣̅1) to point 2 (𝑣̅2)
The change in kinetic energy (KE) is :
∆(KE)=(KE)2-(KE)1=
1
2
m (𝑣̅2
2
-𝑣̅1
2
) [Joule, J]
KE is a property of the body and it’s an extensive property; on an unit mass basis ke (J/kg)
POTENTIAL ENERGY
Body of mass m, moving from point 1(z1) to point 2(z2), in a field with a specified gravity
acceleration (g) value.
The change in potential energy (PE) is:
∆(PE)=(PE)2-(PE)1=m g ( 𝑧2-𝑧1) [Joule, J]
PE is a property of the body and it’s an extensive property; on an unit mass basis pe (J/kg)](https://image.slidesharecdn.com/1lecturenotes1-180430221340/75/1-lecture-notes-1-8-2048.jpg)