Report
Share
Download to read offline
More Related Content
More from handrew
More from handrew (20)
Gr11 Apr8 Mole Mole Calculations
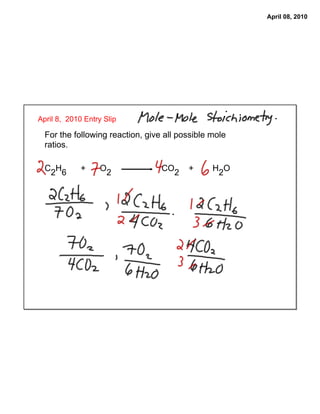
- 1. April 08, 2010 April 8, 2010 Entry Slip For the following reaction, give all possible mole ratios. C2H6 + O2 CO2 + H2O
- 2. April 08, 2010 Determine the number of moles of water produced by the reaction of 0.65 moles of oxygen gas with excess ethane, C2H6. C2H6 + O2 CO2 + H2O Step 1: Make sure the equation is balanced. Step 2: Determine the mole ratio to use. Mole ratio = Unknown Known Step 3: Determine the number of moles of "unknown" required/produced.