Unit-IV_22UCH101_Theory-1.pptx
- 1. 1 UNIT -IV
- 2. 2 QUANTUM THEORY It states that a hot vibrating body does not emit or absorb energy continuously but does so discontinuously in the form of small energy packets or bundles known as quanta or photons in case of light energy. When atoms or molecules absorb or emit radiant energy, they do so in separate ‘units of waves’ called quanta or photons. Thus light radiations obtained from energized or ‘excited atoms’ consist of a stream of photons and not continuous waves (Figure). Figure The energy of radiation (E) is directly proportional to frequency of radiation (𝜈). E ∝ 𝜈 E = h𝜈 = h c/ λ (∵ 𝜈 = c/ λ) Here, h is Planck constant and its value is 6.6253 × 10–34 Js or kg m2 s–l. Absorption or emission of energy in the form of integral multiples of quantum is known as quantization of energy, E = nh𝜈 A hollow sphere coated inside with platinum black and having a small hole in its wall acts as a black body. It is a perfect absorber and perfect emitter of radiant energy.
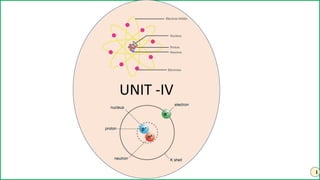
- 3. 3 QUANTUM THEORY AND BOHR ATOM An atom has a positively charged nucleus at its centre and most of the mass of the atom is in the nucleus. The electrons revolve around the nucleus in special orbits called discrete orbits (or) shells (or) stationary state. These orbits are called shells or energy levels and are represented by the letters. These shells are numbered as 1, 2, 3, 4 or termed as K, L, M, N from the nucleus. While revolving in the discrete orbits the electrons do not radiate energy. The centrifugal force of the revolving electron in a stationary orbit is balanced by the electrostatic attraction between the electron and the nucleus. Electron can revolve only in orbits whose angular momentum are an integral multiple of the factor h/2 π. mvr = nh/2π Where m = mass of electron, v = velocity of electron, r = radius of the orbit and ‘n’ is the integral number like, 1, 2, 3, 4 . . . , is called principal quantum number and h = Planck’s constant
- 4. 4 The energy of an electron changes only when it moves from one orbit to another. Outer orbits have higher energies while inner orbits have lower energies. The energy is absorbed when an electron moves from inner orbit to outer orbit. The energy is emitted when the electron jumps from outer orbit to inner orbit. The energy emitted or absorbed in a transition is equal to the difference between the energies of the two orbits (E2 – E1). Energy emitted or absorbed is in the form of quanta. ΔE=E2 – E1 = hν Here E1 and E2 are the lower and higher allowed energy states.
- 5. 5 Bohr, undoubtedly, gave the first quantitative successful model of the atom. The modern Wave Mechanical Theory rejects the view that electrons move in closed orbits, as was visualized by Bohr. The Wave mechanical theory gave a major breakthrough by suggesting that the electron motion is of a complex nature best described by its wave properties and probabilities. The classical ‘mechanical theory’ of matter considered matter to be made of discrete particles (atoms, electrons, protons etc.), The ‘Wave theory’ was necessary to interpret the nature of radiations like X-rays and light. According to the wave theory, radiations as X-rays and light, consisted of continuous collection of waves travelling in space. The wave nature of light, however, failed completely to explain the photoelectric effect i.e. the emission of electron from metal surfaces by the action of light. In their attempt to find a plausible explanation of radiations from heated bodies as also the photoelectric effect. WAVE MECHANICAL CONCEPT OF ATOM
- 6. 6 Planck and Einstein (1905) proposed that energy radiations, including those of heat and light, are emitted discontinuously as little ‘bursts’, quanta, or photons. This view is directly opposed to the wave theory of light and it gives particle-like properties to waves. Light exhibits both a wave and a particle nature, under suitable conditions. This theory which applies to all radiations, is often referred to as the ‘Wave Mechanical Theory’. The Planck’s contention of light having wave and particle nature, the distinction between particles and waves became very hazy. Louis de Broglie advanced a complimentary hypothesis for material particles. According to it, the dual character–the wave and particle–may not be confined to radiations alone but should be extended to matter as well. In other words, matter also possessed particle as well as wave character. This gave birth to the ‘Wave mechanical theory of matter’. This theory postulates that electrons, protons and even atoms, when in motion, possessed wave properties and could also be associated with other characteristics of waves such as wavelength, wave-amplitude and frequency. The new quantum mechanics, which takes into account the particulate and wave nature of matter, is termed the Wave mechanics.
- 7. 7 De Broglie had arrived at his hypothesis with the help of Planck’s Quantum Theory and Einstein’s Theory of Relativity. He derived a relationship between the magnitude of the wavelength associated with the mass ‘m’ of a moving body and its velocity. According to Planck, the photon energy ‘E’ is given by the equation. E = h𝜈 ……………………………….(1) where h is Planck’s constant and ν the frequency of radiation. By applying Einstein’s mass-energy relationship, the energy associated with photon of mass ‘m’ is given as E = mc2………………………………(2) where c is the velocity of radiation comparing equations (1) and (2) mc2 = h𝜈 = h c λ (∵ 𝜈 = 𝑐 𝜆 ) mc = ℎ 𝜆 ………………………………..(3) De BROGLIE’S EQUATION:
- 8. 8 The equation (iii) is called de Broglie’s equation and may be put in words as : The momentum of a particle in motion is inversely proportional to wavelength, Planck’s constant ‘h’ being the constant of proportionality. The wavelength of waves associated with a moving material particle (matter waves) is called de Broglie’s wavelength. The de Broglie’s equation is true for all particles, but it is only with very small particles, such as electrons, that the wave-like aspect is of any significance. Large particles in motion though possess wavelength, but it is not measurable or observable. Let us, for instance consider de Broglie’s wavelengths associated with two bodies and compare their values. Problem 1: What is the de Broglie wavelength? Assume that one mole of protons has a mass equal to 1 g? (h = 6.626 x 10-27 erg s). Problem 2: An electron is moving with a kinetic energy of 4.55 x 10–25 J. What will be de Broglie wavelength for this electron?
- 9. 9 Heisenberg’s uncertainty principle is applicable only to subatomic particles. According to this principle, it is impossible to measure simultaneously and accurately both change in position and change in momentum. If the value of one is determined with certainty, the accuracy in determining the other value is compromised. It is also called principle of indeterminacy. Δx. Δp ≥ ℎ 4𝜋 Δx. mΔv ≥ ℎ 4𝜋 Here Δx = Uncertainty in position Δv= Uncertainty in velocity Δx. Δv ≥ h 4πm ΔE. Δt ≥ h 4π Here ΔE = change in energy Δt = Change in time If change in position is zero, change in momentum will be infinite and vice versa. It applies when both Δx and Δp are along the same axis. Concept of Probability: As a according to this principle Δx and ΔP of an e– can not be find accurately at a particular time so the idea of definite paths i.e., orbits as suggested by Bohr has no more significance. This leads to the concept of probability. According to which it is possible to state or predict the probability of locating an electron of a specific energy in a given region of space around the nucleus at a given time. This leads to the concept of ‘orbital’. HEISENBERG’S UNCERTAINTY PRINCIPLE
- 10. 10 Schrödinger derived an equation known after his name as Schrödinger’s Wave Equation. Calculation of the probability of finding the electron at various points in an atom was the main problem before Schrödinger. Schrödinger equation is the keynote of wave mechanics and is based upon the idea of the electron as ‘standing wave’ around the nucleus. The equation for the standing wave, comparable with that of a stretched string is ………………(a) (i) where ψ (pronounced as sigh) is a mathematical function representing the amplitude of wave (called wave function) (ii) x, the displacement in a given direction, and (iii) λ, the wavelength and (iv) A is a constant. By differentiating equation (a) twice with respect to x, we get ………………(1) ………………(2) and ………………(3) But ∴ SCHRÖDINGER’S WAVE EQUATION
- 11. 11 The K.E. of the particle of mass m and velocity ν is given by the relation ………………(4) According to Broglie’s equation or or Substituting the value of m2 v2, we have ………………(5) From equation (3), we have ………………(6) Substituting the value of λ2 in equation (5)
- 12. 12 The total energy E of a particle is the sum of kinetic energy and the potential energy i.e., or or This is Schrödinger’s equation in one dimension. It need be generalized for a particle whose motion is described by three space coordinates x, y and z. Thus,
- 13. 13 This equation is called the Schrödinger’s Wave Equation. The first three terms on the left-hand side are represented by Δ2ψ (pronounced as del-square sigh). Δ2 is known as Laplacian Operator. The Schrödinger’s wave equation is a second degree differential equation. It has several solutions. Some of these are imaginary and are not valid. If the potential energy term is known, the total energy E and the corresponding wave function ψ can be evaluated. The wave function is always finite, single valued and continuous. It is zero at infinite distance. Solutions that meet these requirements are only possible if E is given certain characteristic values called Eigen-values. Corresponding to these values of E, we have several characteristic values of wavefunction ψ and are called Eigen-functions. As the eigen-values correspond very nearly to the energy values associated with different Bohr-orbits, the Bohr’s model may be considered as a direct consequence of wave mechanical approach.
- 14. 14 ψ : It has no physical significance. It represents amplitude of the spherical electron-wave or boundary surface of an orbital. ψ2: It is the probable electron density or it is the probability of finding electrons in any region (3 dimensional space around the nucleus). If ψ2 is positive, electrons are present and if ψ2 is zero electrons are absent. An orbital is represented by ψ, ψ* or ψ2 for showing electron density. For hydrogen atom, Schrödinger’s Wave Equation gives the wave function of the electron (with energy = – 2.18 × 10–11 ergs) situated at a distance ‘r’, ψ = C1e – C2r , where C1 and C2 are constants. The square of the amplitude ψ2 is proportional to the density of the wave. The wave of energy or the cloud of negative charge is denser in some parts than in others. Max Born interpreted the wave equations on the basis of probabilities. Even if an electron be considered as a particle in motion around the nucleus, the wave equation may be interpreted in terms of probability or relative chance of finding the electron at any given distance from the nucleus. The space characteristic of an electron is best described in terms of distribution function given by D = 4πr2 ψ2 The numerical value of ‘D’ denotes the probability or chance of finding the electron in a shell of radius r and thickness dr, or of volume 4πr2 dr. Significance of ψ and ψ2
- 15. 15 Substituting for ψ we have, The probability of finding the electron is clearly a function of ‘r’. When r = 0 or ∝ , the probability function D becomes equal to zero. In other words, there is no probability of finding the electron at the nucleus or at infinity. However, it is possible to choose a value of r such that there is 90- 95 percent chance of finding the electron at this distance. For the hydrogen atom, this distance is equal to 0.53 × 10 cm or 0.53 Å. If the probability distribution be plotted against the distance r from the nucleus, the curve obtained is shown in Figure The probability distribution is maximum at the distance 0.53 Å and spherically symmetrical. This distance corresponds to Bohr’s first radius a0. The graph can be interpreted as representing a contour that encloses a high-percentage of charge. When the electron gets excited and it is raised from n to higher energy levels (say n = 2 or n = 3), the solution of wave equation gives sets of value of ψ2 which give different shapes to the space distribution of the electron. Figure Shows the probability distribution of electron cloud : (a) gives the graphical representation while (b) depicts cross-section of the cloud.
- 16. 16 The probability of finding electron at a distance r from the nucleus in the region dr is given by 4 π r2 dr ψ*. In case where the energy of an atom or a molecule does not change, another equation given by Schrodinger becomes applicable. H ψ = E ψ H = T + V (T + V) ψ = E ψ Here, H = Hamiltonian factor V = Potential energy T = Kinetic energy E = Total energy
- 17. 17 CHARGE CLOUD CONCEPT AND ORBITALS The Charge Cloud Concept finds its birth from wave mechanical theory of the atom. The wave equation for a given electron, on solving gives a three-dimensional arrangement of points where it can possibly lie. There are regions where the chances of finding the electron are relatively greater. Such regions are expressed in terms of ‘cloud of negative charge’. We need not know the specific location of the electrons in space but are concerned with the negative charge density regions. Electrons in atoms are assumed to be vibrating in space, moving haphazardly but at the same time are constrained to lie in regions of highest probability for most of the time. The charge cloud concept simply describes the high probability region. The three-dimensional region within which there is higher probability that an electron having a certain energy will be found, is called an orbital. An orbital is the most probable space in which the electron spends most of its time while in constant motion. In other words, it is the spatial description of the motion of an electron corresponding to a particular energy level. The energy of electron in an atomic orbital is always the same. Each energy level corresponds to a three-dimensional electron wave which envelopes the nucleus. This wave possesses a definite ‘size’, ‘shape’ and ‘orientation’ and thus can be represented pictorially.
- 19. 19 QUANTUM NUMBERS Quantum numbers work like addresses for electrons in an atom. To narrow down your intended recipient, you would write the state →city → street → street number to tell the post office where to go. Quantum numbers work the same way: energy level → type of orbital → orientation of orbital → orientation of electron. Every electron in an atom will have a unique set of quantum numbers in the form [n, l, ml, ms]. Quantum numbers are set of four numbers used to address an electron as follows: To determine size, distance from nucleus and energy of electron in orbit To decide shape, energy of electron in a sub-orbit. To find number of orbitals and their directional position or orientation in space. To find number of electrons and their spin. Principle Quantum Number Principle quantum number was introduced by Bohr. It is denoted by n. It determines: Size of orbit (shell)
- 20. 20 Angular momentum of electron in an orbit mvr = angular momentum in orbit. h = Planck constant Values of n = 1,2 ,3, 4 ............... ∞ n ≠ 0, –ve, fractional Azimuthal or Secondary or Angular Momentum Quantum Number Angular momentum quantum number was introduced by Sommerfield. It is denoted by ‘l’. It determines shape of sub-orbit, energy in sub shell and angular momentum of an electron in any orbital. This explains the existence of many closely packed spectral lines in hydrogen spectrum. Values of l = 0 to n – 1
- 21. 21 Magnetic Quantum Number Magnetic quantum number was introduced by Land and Zeeman. It is denoted by m. It determines: Number of orbitals m (total) value = n2 (when n is given) = 2l + 1 when ‘l’is given. when n = 2 m (total) = 22 = 4 when l = 2 m (total) = 2 × 2 + 1 = 5 Directional position of orbitals or orientation in shape and Zeemann effect Angular magnetic momentum Number of orbital in a subshell = (2l + 1) Maximum number of electrons in a particular orbital in a subshell = 2(2l + 1) = 4l + 2 Values of m = –l to + l including zero
- 22. 22 Spin quantum number was introduced by Uhlenbeck and Goldsmith. It is the intrinsic angular momentum measurement which is used to differentiate between two electrons in the same orbital. Schrodinger could not explain the spin of an electron. For each value of m, s has two different values + 1/2 and –1/2. +1/2 –1/2 , α-spin, β-spin +1/2 and –1/2 are just to mechanical spin states with no classical importance now as other spin states are also possible. Spin Quantum Number
- 23. 23
- 24. 24
- 25. 25 ENERGY DISTRIBUTION AND ORBITALS Hydrogen and Hydrogen-like atoms: Hydrogen atom : In case of hydrogen atom, energy of orbital is mainly determined by principle quantum number. FILLING OF ORBITALS IN ATOMS : Pauli exclusion principle—Any molecular orbital can have a maximum of two electrons with opposite spin. Hund’s rule—In degenerate molecular orbital, before pairing, each molecular orbital must have one electron. Aufbau principle—Electrons are filled from molecular orbital of lower energy to higher energy.
- 26. 26 GROUND STATE ELECTRONIC CONFIGURATION