Report
Share
Download to read offline
Recommended
Recommended
Health Canada Progressive Licensing - Prharmaceuticals
#peivandpirouzi #training #canada #pirouzi #international #funding #immigrants #refugees #canada #immigration #education Health Canada Progressive Licensing - Professor Peivand Pirouzi

Health Canada Progressive Licensing - Professor Peivand PirouziPharmaceutical Compliance Inspection unit, Crown College of Canada
Expert Patient Advocates & 21st Century Therapies Forum Nov 19 - 20, 2015Maureen smith patient submissionspresentation.m smith.nov2015

Maureen smith patient submissionspresentation.m smith.nov2015Canadian Organization for Rare Disorders
A Rare International Dialogue (Saturday May 11, 2019)
Drivers of Drug Development – Regulatory Collaboration
Medical Product Development for Rare Diseases: FDA perspective CDER Larissa Lapteva, US Food and Drug Administration, CBER Medical Product Development For Rare Diseases
Part 2: Regenerative Medicine Therapies
FDA Regulatory PerspectiveSTREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...

STREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...Canadian Organization for Rare Disorders
More Related Content
What's hot
Health Canada Progressive Licensing - Prharmaceuticals
#peivandpirouzi #training #canada #pirouzi #international #funding #immigrants #refugees #canada #immigration #education Health Canada Progressive Licensing - Professor Peivand Pirouzi

Health Canada Progressive Licensing - Professor Peivand PirouziPharmaceutical Compliance Inspection unit, Crown College of Canada
Expert Patient Advocates & 21st Century Therapies Forum Nov 19 - 20, 2015Maureen smith patient submissionspresentation.m smith.nov2015

Maureen smith patient submissionspresentation.m smith.nov2015Canadian Organization for Rare Disorders
A Rare International Dialogue (Saturday May 11, 2019)
Drivers of Drug Development – Regulatory Collaboration
Medical Product Development for Rare Diseases: FDA perspective CDER Larissa Lapteva, US Food and Drug Administration, CBER Medical Product Development For Rare Diseases
Part 2: Regenerative Medicine Therapies
FDA Regulatory PerspectiveSTREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...

STREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...Canadian Organization for Rare Disorders
What's hot (20)
Connecting the Dots for Fast-Track Approval for Rare Disease and Orphan Drug

Connecting the Dots for Fast-Track Approval for Rare Disease and Orphan Drug
Health Canada Progressive Licensing - Professor Peivand Pirouzi

Health Canada Progressive Licensing - Professor Peivand Pirouzi
Safety monitoring and reporting of adverse events of medical devices national...

Safety monitoring and reporting of adverse events of medical devices national...
Maureen smith patient submissionspresentation.m smith.nov2015

Maureen smith patient submissionspresentation.m smith.nov2015
Pharmacovigilance Training in Oracle Argus Safety Database

Pharmacovigilance Training in Oracle Argus Safety Database
Pharmacovigilance Establishment in India and An overview on PvPI

Pharmacovigilance Establishment in India and An overview on PvPI
STREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...

STREAM TWO: Larissa Lapteva, Medical Product Development For Rare Diseases Pa...
Similar to Poster plantas para entregar
Herbal products, also known as botanical products or phytomedicines, are products made from plants to treat diseases or maintain health.
Herbal products are made by extracting active ingredients from plant parts, such as leaves, bark, roots, seeds, or flowers.
Herbal products, including dietary supplements, are regulated by the FDA. However, unlike pharmaceutical drugs, they do not require pre-market approval. Instead, manufacturers are responsible for ensuring the quality and safety of their products.
The FDA establishes Good Manufacturing Practices (GMP) regulations for dietary supplements to ensure quality control during manufacturing.
The safety of herbal products in the USA is overseen by various regulatory agencies, primarily the Food and Drug Administration (FDA) and the Federal Trade Commission (FTC).
To ensure a high degree of safety and effectiveness of herbal products and quality control standards during the manufacturing of herbal supplements and medicines, AHPA published GACP (Good Agriculture and Collection Practices) guideline in the American Herbal Pharmacopoeia.
Quality, Safety and Legislation for herbal products in USA.pptx

Quality, Safety and Legislation for herbal products in USA.pptxNitte Gulabi Shetty Memorial Institute of Pharmaceutical Sciences
Similar to Poster plantas para entregar (20)
Quality, Safety and Legislation for herbal products in USA.pptx

Quality, Safety and Legislation for herbal products in USA.pptx
Poster plantas para entregar
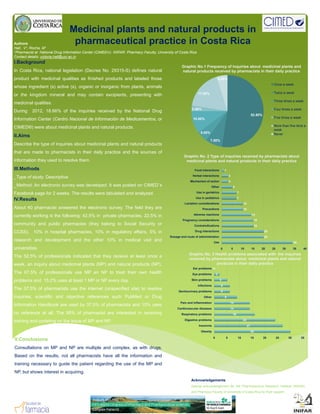
- 1. Medicinal plants and natural products in pharmaceutical practice in Costa RicaAuthors Hall, V1; Rocha, M1 1Pharmacist at National Drug Information Center (CIMED®) INIFAR Pharmacy Faculty University of Costa Rica1Pharmacist at National Drug Information Center (CIMED®). INIFAR. Pharmacy Faculty, University of Costa Rica Contact details: victoria.hall@ucr.ac.cr I.Background In Costa Rica, national legislation (Decree No. 29315-S) defines natural product with medicinal qualities as finished products and labeled those whose ingredient (s) active (s), organic or inorganic from plants, animals or the kingdom mineral and may contain excipients, presenting with medicinal qualities 17.50% 5.00% Once a week Twice a week Three times a week Graphic No.1 Frequency of inquiries about medicinal plants and natural products received by pharmacists in their daily practice medicinal qualities. During 2012, 18.66% of the inquiries received by the National Drug Information Center (Centro Nacional de Información de Medicamentos, or CIMED®) were about medicinal plants and natural products. II.Aims Describe the type of inquiries about medicinal plants and natural products that are made to pharmacists in their daily practice and the sources of 52.50% 7.50% 5.00% 10.00% 2.50% Four times a week Five times a week More than five time a week Never G hi N 2 T f i i i i d b h i t b t information they used to resolve them. III.Methods _Type of study: Descriptive _Method: An electronic survey was developed. It was posted on CIMED´s Facebook page for 2 weeks. The results were tabulated and analyzed. IV.Results About 40 pharmacist answered the electronic survey The field they are 10 7 7 5 3 3 1 Lactation considerations Use in pediatrics Use in geriatrics Other Mechanism of action Herbal interactions Food interactions Graphic No. 2 Type of inquiries received by pharmacists about medicinal plants and natural products in their daily practice About 40 pharmacist answered the electronic survey. The field they are currently working is the following: 42.5% in private pharmacies, 22.5% in community and public pharmacies (they belong to Social Security or CCSS), 10% in hospital pharmacies, 10% in regulatory affairs, 5% in research and development and the other 10% in medical visit and universities. The 52.5% of professionals indicated that they receive at least once a 34 20 20 15 15 14 10 0 5 10 15 20 25 30 35 40 Use Dosage and route of administration Drug interactions Contraindications Pregnancy considerations Adverse reactions Precautions Graphic No. 3 Health problems associated with the inquiries received by pharmacists about medicinal plants and natural week, an inquiry about medicinal plants (MP) and natural products (NP). The 67.5% of professionals use MP an NP to treat their own health problems and 15.2% uses at least 1 MP or NP every day. The 37.5% of pharmacists use the internet (unspecified site) to resolve inquiries; scientific and objective references such PubMed or Drug Information Handbook are used by 37.5% of pharmacists and 10% uses 14 14 9 6 6 5 2 0 Cardiovascular diseases Pain and inflammation Other Genitourinary problems Infections Skin problems Eye problems Ear problems received by pharmacists about medicinal plants and natural products in their daily practice no reference at all. The 95% of pharmacist are interested in receiving training and updating on the issue of MP and NP. V.Conclusions Consultations on MP and NP are multiple and complex, as with drugs. Based on the results, not all pharmacists have all the information and t i i t id th ti t di th f th MP d 30 27 24 16 0 5 10 15 20 25 30 35 Obesity Insomnia Digestive problems Respiratory problems training necessary to guide the patient regarding the use of the MP and NP, but shows interest in acquiring. Acknowledgements Special acknowledgement for the Pharmaceutical Research Institute (INFAR) and Pharmacy Faculty of University of Costa Rica for their support.
