QA &QC.pdf
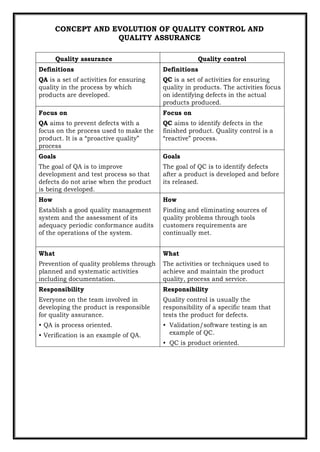
- 1. CONCEPT AND EVOLUTION OF QUALITY CONTROL AND QUALITY ASSURANCE Quality assurance Quality control Definitions QA is a set of activities for ensuring quality in the process by which products are developed. Definitions QC is a set of activities for ensuring quality in products. The activities focus on identifying defects in the actual products produced. Focus on QA aims to prevent defects with a focus on the process used to make the product. It is a “proactive quality” process Focus on QC aims to identify defects in the finished product. Quality control is a “reactive” process. Goals The goal of QA is to improve development and test process so that defects do not arise when the product is being developed. Goals The goal of QC is to identify defects after a product is developed and before its released. How Establish a good quality management system and the assessment of its adequacy periodic conformance audits of the operations of the system. How Finding and eliminating sources of quality problems through tools customers requirements are continually met. What Prevention of quality problems through planned and systematic activities including documentation. What The activities or techniques used to achieve and maintain the product quality, process and service. Responsibility Everyone on the team involved in developing the product is responsible for quality assurance. • QA is process oriented. • Verification is an example of QA. Responsibility Quality control is usually the responsibility of a specific team that tests the product for defects. • Validation/software testing is an example of QC. • QC is product oriented.
- 2. ICH The international council for harmonization of technical requirements for pharmaceuticals for human use is unique in bringing together the regulatory authorities and pharmaceutical industry to discuss scientific and technical aspects of pharmaceuticals and develop ICH guidelines Quality guidelines Safety guidelines Efficacy guidelines Multidisciplinary Quality guidelines: Harmonization achievements in the quality area include pivotal milestones such as conduct of stability studies, defining relevant thresholds for impurities testing and a more flexible approach to pharmaceutical quality based on good manufacturing practice (GMP) risk management. 𝐐𝟏𝐀 - 𝐐𝟏𝐅 Stability:- 𝑸𝟏𝑨 (𝑹𝟐) - Stability testing of new drug substances and products. 𝑸𝟏𝑩 - Stability testing: photo stability 𝑸𝟏𝑪 - Stability testing for new dosage forms 𝑸𝟏𝑫 - Bracketing and matrixing designs for stability 𝑸𝟏𝑬 - Evaluation of stability data 𝑸𝟏𝑭 - Stability data package for registration applications in climatic zones iii and iv 𝑸𝟐- Analytical validation (𝑹𝟏) − Validation of analytical procedures: text and methodology (𝑹𝟐)/𝑸𝟏𝟒 EWG - Analytical procedure development and revision of (𝑹𝟏) analytical validation 𝑸𝟑𝑨 − 𝑸𝟑𝑫 Impurities (𝑹𝟐) − Impurities in new drug substances (𝑹𝟐) − Impurities in new drug products (𝑹𝟔) − Maintenance of the guidelines for residual solvents
- 3. (𝑹𝟖) − Maintenance EWG maintenance of the guideline for residual solvents (𝑹𝟏) − Guidelines for elemental impurities (𝑹𝟐) − EWG maintenance revision of 𝑸𝟑(𝑹𝟏)for cutaneous and transdermal products 𝑸𝟑𝑫 − Training implementation of guidelines for elemental impurities 𝑸𝟑𝑬 𝑰𝒏𝒇𝒐𝒓𝒎𝒂𝒍 𝑾𝑮 − Impurity: Assessment and control of extractables and leachables for pharmaceuticals and biological 𝑸𝟒𝑨 − 𝑸𝟒𝑩 Pharmacopoeias 𝑸𝟒𝑨 − Pharmacopoeial harmonization 𝑸𝟒𝑩 − Evaluation and recommendation of pharmacopoeial texts for use in the ICH regions 𝑸𝟒𝑩Annex 1(𝑹𝟏) − Residue on ignition/ sulphated ash general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Test for extractable volume of parenteral preparations general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Test for particulate contamination: sub- visible particles general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Microbiological examination of non- sterile products: Microbiological enumeration tests general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Microbiological examination of non- sterile products: tests for specified micro-organisms general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Microbiological examination of non- sterile products: Acceptance criteria for pharmaceutical preparation and substances for pharmaceutical use general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Disintegration test general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟔 − Uniformity of dosage unit’s general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟐) − Dissolution test general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟖 (𝑹𝟏) − Sterility test general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 (𝑹𝟏) − Tablet friability general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟏𝟎 (𝑹𝟏) − Polyacrylamide gel electrophoresis general chapter
- 4. 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟏𝟏 − Capillary electrophoresis general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟏𝟐 − Analytical sieving general chapter 𝑸𝟒𝑩 𝑨𝒏𝒏𝒆𝒙 𝟏𝟑 − Bulk density and tapped density of powders general chapter 𝑸𝟒𝑩 𝑨𝑵𝑵𝑬𝑿: 𝟏𝟒 − Bacterial endotoxins test general chapter 𝐐𝟒𝐁 FAQS - Frequently asked questions 𝑸𝟓𝑨 − 𝑸𝟓 𝑬 Quality of biotechnologicalproducts 𝑸𝟓𝑨 (𝑹𝟏) - Viral safety evaluation of biotechnology products derived from cell lines of human (or) animal origin 𝑸𝟓𝑨 (𝑹𝟐) EWG - Viral safety evaluation of biotechnology products derived from cell lines of human (or) animal origin 𝑸𝟓𝑩 - Analysis of the expression construct in cells used for production of r- DNA derived protein products 𝑸𝟓𝑪 - Quality of biotechnological products: Stability testing of biotechnological/biological products 𝑸𝟓𝑫 - Derivation and characterization of cell substances used for production of biotechnological/biological products 𝑸𝟓𝑬 - Comparability of biotechnological/biological products subject to changes in their manufacturing process 𝑸𝟔𝑨 − 𝑸𝟔𝑩- Specifications 𝑸𝟔𝑨 - Specifications: Test procedures and acceptance criteria for new drug substances and new drug product: chemical substances. 𝑸𝟔𝑩 - Specifications: Test procedures and acceptance criteria for biotechnological/biological products 𝑸𝟕 - Good manufacturing practice 𝑸𝟕 - Good manufacturing practice guidelines for active pharmaceutical ingredients 𝐐𝟕 Q&AS - Questions and answers: Good manufacturing practice for active pharmaceutical ingredients 𝑸𝟖 - Pharmaceutical development
- 5. (𝑹𝟐) - Pharmaceutical development 𝐐𝟖/𝐐𝟗/𝐐𝟏𝟎Q&AS (𝑹𝟒) 𝑸𝟖/𝑸𝟗/ 𝑸𝟏𝟎 - Implementation 𝑸𝟗-Quality Risk management 𝐐𝟖/𝐐𝟗/ 𝐐𝟏𝟎 Q&AS (𝑹𝟒)/𝐐𝟗/𝐐𝟏𝟎 – Implementation 𝑸𝟏𝟎 – Pharmaceutical quality system 𝑸𝟖/𝑸𝟗/(𝑹𝟒) − Implementation 𝐐𝟏𝟏 - Development and manufacture of drug substances (chemical entities and biotechnological/biological entities) 𝐐𝟏𝟐 − Life cyclemanagement 𝐐𝟏𝟐 EWG – Technical and regulatory considerations for pharmaceutical product life cycle management 𝐐𝟏𝟑 - Continuous manufacturing of drug substances and drug products 𝐐𝟏𝟑 EWG - Continuous manufacturing of drug substances and drug products 𝑸𝟏𝟒 - Analytical proceduredevelopment 𝑸𝟐 (𝑹𝟐) /𝑸𝟏𝟒 EWG - Analytical procedure development and revision of analytical validation Good laboratory practices Definition: GLP is a set of principles that provides a frame work intended to assure the quality and clinical laboratory studies that are planned, performed, monitored, archived, and reported to support research or marketing permits for products regulated by Government agencies. The term GLP is most commonly associated with the pharmaceutical industry and the required non clinical animal testing that must be performed prior to approval of new drug products However, GLP applies to many other non-pharmaceutical agents such as food additives, color additives, food contamination limits, food packaging and medical device. This GLP comes under code of federal regulations by USFDA in title 21 which means it is for food and drugs under this part 58 is GLP. • GLP under part 58 have subparts from A to K. • Subpart A- General provisions • Subpart B- Organization and personnel
- 6. • Subpart C- Facilities • Subpart D- Equipment • Subpart E- Testing facilitiesoperation • Subpart F- Test and control articles • Subpart G- Protocol for and conduct of a nonclinical laboratory study • Subpart H-I- Reserved • Subpart J- Records and Reports • Subpart K- Disqualification of testing facilities. Subpart- A General provisions: Scope Definitions Applicability to studies performed under grants and contracts Inspection of a testing facility. Scope: This part prescribes good laboratory practices for conducting nonclinical laboratory studies that support or are intended to support applications for research or marketing permits for products regulated by the Food and Drug Administration, including food and color additives, animal food additives, human and animal drugs, medical devices for human use, biological products, and electronic products. Compliance with this part is intended to assure the quality and integrity of the safety data filed pursuant to sections 406, 408, 409, 502, 503, 505, 506, 510, 512-516, 518-520, 721, and 801 of the Federal Food, Drug, and Cosmetic Act and sections 351 and 354-360F of the Public Health Service Act. Definitions: Test article means any food additive, color additive, drug, biological product, electronic product, medical device for human use, or any other article subject to regulation under the act or under sections 351 and 354-360F of the Public Health Service Act. Control article means any food additive, color additive, drug, biological product, electronic product, medical device for human use, or any article other than a test article, feed, or water that is administered to the test system in the course of a nonclinical laboratory study for the purpose of establishing a basis for comparison with the test article. Nonclinical laboratory study means in vivo and in vitro experiments in which test articles are studied prospectively in test systems under laboratory conditions to determine their safety. The term does not include studies utilizing human subjects or clinical studies or field trials in animals. The term does not include basic exploratory studies carried out to determine whether a test article has any potential utility or to determine physical or chemical characteristics of a test article. Sponsor means: 1. A person who initiates and supports, by provision of financial or other
- 7. resources, a nonclinical laboratory study; 2. A person who submits a nonclinical study to the Food and Drug Administration in support of an application for a research or marketing permit. • Testing facility means a person who actually conducts a nonclinical laboratory study, i.e., actually uses the test article in a test system. • Test system means any animal, plant, microorganism, or subparts thereof to which the test or control article is administered or added for study. • Specimen means any material derived from a test system for examination or analysis • Raw data means any laboratory worksheets, records, memoranda, notes, or exact copies thereof, that are the result of original observations and activities of a nonclinical laboratory study and are necessary for the reconstruction and evaluation of the report of that study. • Quality assurance unit means any person or organizational element, except the study director, designated by testing facility management to perform the duties relating to quality assurance of nonclinical laboratory studies. • Study director means the individual responsible for the overall conduct of a nonclinical laboratory study. • Batch means a specific quantity or lot of a test or control article that has been characterized. • Study initiation date means the date the protocol is signed by the study director. • Study completion date means the date the final report is signed by the study director. Applicabilityto studies performed under grants and contracts: When a sponsor conducting a nonclinical laboratory study intended to be submitted to or reviewed by the Food and Drug Administration utilizes the services of a consulting laboratory, contractor, or grantee to perform an analysis or other service, it shall notify the consulting laboratory, contractor, or grantee that the service is part of a nonclinical laboratory study that must be conducted in compliance with the provisions of this part. Inspection of a testing facility A testing facility shall permit an authorized employee of the Food and Drug Administration, at reasonable times and in a reasonable manner, to inspect the facility and to inspect (and in the case of records also to copy) all records and specimens required to be maintained regarding studies within the scope of this part. The records inspection and copying requirements shall not apply to quality assurance unit records of findings and problems, or to actions recommended and taken. Subpart – B organization and personnel Each individual engaged in the conduct of or responsible for the
- 8. supervision of a nonclinical laboratory study shall have education, training, and experience, or combination thereof, to enable that individual to perform the assigned functions. Each testing facility shall maintain a current summary of training and experience and job description for each individual engaged in or supervising the conduct of a nonclinical laboratory study. There shall be a sufficient number of personnel for the timely and proper conduct of the study according to the protocol. Personnel shall take necessary personal sanitation and health precautions designed to avoid contamination of test and control articles and test systems. Personnel engaged in a nonclinical laboratory study shall wear clothing appropriate for the duties they perform. Such clothing shall be changed as often as necessary to prevent microbiological, radiological, or chemical contamination of test systems and test and control articles. Any individual found at any time to have an illness that may adversely affect the quality and integrity of the nonclinical laboratory study shall be excluded from direct contact with test systems, test and control articles and any other operation or function that may adversely affect the study until the condition is corrected. All personnel shall be instructed to report to their immediate supervisors any health or medical conditions that may reasonably be considered to have an adverse effect on a nonclinical laboratory study. Testing facility management For each nonclinical laboratory study, testing facility management shall: a. Designate a study director as described in §58.33, before the study is initiated. b. Replace the study director promptly if it becomes necessary to do so during the conduct of a study. c. Assure that there is a quality assurance unit as described in §58.35. d. Assure that test and control articles or mixtures have been appropriately tested for identity, strength, purity, stability, and uniformity, as applicable. e. Assure that personnel, resources, facilities, equipment, materials, and methodologies are available as scheduled. f. Assure that personnel clearly understand the functions they are to perform. g. Assure that any deviations from these regulations reported by the quality assurance unit are communicated to the study director and corrective actions are taken and documented. Study director For each nonclinical laboratory study, a scientist or other professional of appropriate education, training, and experience, or combination thereof, shall be identified as the study director. The study director has overall responsibility for the technical conduct of the study, as well as for the interpretation, analysis, documentation and reporting of results, and represents the single point of study control. The study director shall assure that:
- 9. a. The protocol, including any change, is approved as provided by §58.120 and is followed. b. All experimental data, including observations of unanticipated responses of the test system are accurately recorded and verified. c. Unforeseen circumstances that may affect the quality and integrity of the nonclinical laboratory study are noted when they occur, and corrective action is taken and documented. d.Test systems are as specified in the protocol. e. All applicable good laboratory practice regulations are followed. f. All raw data, documentation, protocols, specimens, and final reports are transferred to the archives during or at the close of the study. Quality assurance unit A. A testing facility shall have a quality assurance unit which shall be responsible for monitoring each study to assure management that the facilities, equipment, personnel, methods, practices, records, and controls are in conformance with the regulations in this part. B. The quality assurance unit shall: 1. Maintain a copy of a master schedule sheet of all nonclinical laboratory studies conducted at the testing facility indexed by test article and containing the test system, nature of study, date study was initiated, current status of each study, identity of the sponsor, and name of the study director. 2. Maintain copies of all protocols pertaining to all nonclinical laboratory studies for which the unit is responsible. Inspect each nonclinical laboratory study at intervals adequate to assure the integrity of the study and maintain written and properly signed records of each periodic inspection showing the date of the inspection, the study inspected, the phase or segment of the study inspected, the person performing the inspection, findings and problems, action recommended and taken to resolve existing problems, and any scheduled date for re inspection. Any problems found during the course of an inspection which are likely to affect study integrity shall be brought to the attention of the study director and management immediately. 3. Periodically submit to management and the study director written status reports on each study, noting any problems and the corrective actions taken. 4. Determine that no deviations from approved protocols or standard operating procedures were made without proper authorization and documentation. 5. Review the final study report to assure that such report accurately describes the methods and standard operating procedures, and that the reported results accurately reflect the raw data of the nonclinical laboratory study. 6. Prepare and sign a statement to be included with the final study report which shall specify the dates inspections were made and findings reported to management and to the study director.
- 10. C. The responsibilities and procedures applicable to the quality assurance unit, the records maintained by the quality assurance unit, and the method of indexing such records shall be in writing and shall be maintained. These items including inspection dates, the study inspected, the phase or segment of the study inspected, and the name of the individual performing the inspection shall be made available for inspection to authorized employees of the Food and Drug Administration. D. A designated representative of the Food and Drug Administration shall have access to the written procedures established for the inspection and may request testing facility management to certify that inspections are being implemented, performed, documented, and followed-up in accordance with this paragraph. Subpart C-Facilities General Each testing facility shall be of suitable size and construction to facilitate the proper conduct of nonclinical laboratory studies. It shall be designed so that there is a degree of separation that will prevent any function or activity from having an adverse effect on the study. Animal care facilities A. A testing facility shall have a sufficient number of animal rooms or areas, as needed, to assure proper: 1. Separation of species or test systems. 2. Isolation of individual projects. 3. Quarantine of animals. 4. Routine or specialized housing of animals. B. A testing facility shall have a number of animal rooms or areas separate from those described in paragraph (a) of this section to ensure isolation of studies being done with test systems or test and control articles known to be biohazardous, including volatile substances, aerosols, radioactive materials, and infectious agents. C. Separate areas shall be provided, as appropriate, for the diagnosis, treatment, and control of laboratory animal diseases. These areas shall provide effective isolation for the housing of animals either known or suspected of being diseased, or of being carriers of disease, from other animals. D. When animals are housed, facilities shall exist for the collection and disposal of all animal waste and refuse or for safe sanitary storage of waste before removal from the testing facility. Disposal facilities shall be so provided and operated as to minimize vermin infestation, odors, disease hazards, and environmental contamination. Animal supply facilities There shall be storage areas, as needed, for feed, bedding, supplies, and equipment. Storage areas for feed and bedding shall be separated from areas housing the test systems and shall be protected against infestation or
- 11. contamination. Perishable supplies shall be preserved by appropriate means. Facilities for handling test and control articles A. As necessary to prevent contamination or mixups, there shall be separate areas for: 1. Receipt and storage of the test and control articles. 2. Mixing of the test and control articles with a carrier, e.g., feed. 3. Storage of the test and control article mixtures. B. Storage areas for the test and/or control article and test and control mixtures shall be separate from areas housing the test systems and shall be adequate to preserve the identity, strength, purity, and stability of the articles and mixtures. Laboratory operation areas Separate laboratory space shall be provided, as needed, for the performance of the routine and specialized procedures required by nonclinical laboratory studies. Specimen and data storage facilities Space shall be provided for archives, limited to access by authorized personnel only, for the storage and retrieval of all raw data and specimens from completed studies. Subpart D - Equipment Equipment design Equipment used in the generation, measurement, or assessment of data and equipment used for facility environmental control shall be of appropriate design and adequate capacity to function according to the protocol and shall be suitably located for operation, inspection, cleaning, and maintenance. Maintenance and calibration of equipment A. Equipment shall be adequately inspected, cleaned, and maintained. Equipment used for the generation, measurement, or assessment of data shall be adequately tested, calibrated and/or standardized. B. The written standard operating procedures required under §58.81(b)(11) shall set forth in sufficient detail the methods, materials, and schedules to be used in the routine inspection, cleaning, maintenance, testing, calibration, and/or standardization of equipment, and shall specify, when appropriate, remedial action to be taken in the event of failure or malfunction of equipment. The written standard operating procedures shall designate the person responsible for the performance of each operation. C. Written records shall be maintained of all inspection, maintenance, testing, calibrating and/or standardizing operations. These records, containing the date of the operation, shall describe whether the
- 12. maintenance operations were routine and followed the written standard operating procedures. Written records shall be kept of non-routine repairs performed on equipment as a result of failure and malfunction. Such records shall document the nature of the defect, how and when the defect was discovered, and any remedial action taken in response to the defect. Subpart E - Testing Facilities Operation Standard operating procedures A. A testing facility shall have standard operating procedures in writing setting forth nonclinical laboratory study methods that management is satisfied are adequate to insure the quality and integrity of the data generated in the course of a study. All deviations in a study from standard operating procedures shall be authorized by the study director and shall be documented in the raw data. Significant changes in established standard operating procedures shall be properly authorized in writing by management. B. Standard operating procedures shall be established for, but not limited to, the following: 1. Animal room preparation. 2. Animal care. 3. Receipt, identification, storage, handling, mixing, and method of sampling of the test and control articles. 4. Test system observations. 5. Laboratory tests. 6. Handling of animals found moribund or dead during study. 7. Necropsy of animals or postmortem examination of animals. 8. Collection and identification of specimens. 9. Histopathology. 10. Data handling, storage, and retrieval. 11. Maintenance and calibration of equipment. 12. Transfer, proper placement, and identification of animals. C. Each laboratory area shall have immediately available laboratory manuals and standard operating procedures relative to the laboratory procedures being performed. Published literature may be used as a supplement to standard operating procedures. D. A historical file of standard operating procedures, and all revisions thereof, including the dates of such revisions, shall be maintained. Reagents and solutions All reagents and solutions in the laboratory areas shall be labeled to indicate identity, titer or concentration, storage requirements, and expiration date. Deteriorated or outdated reagents and solutions shall not be used. Animal care A. There shall be standard operating procedures for the housing, feeding, handling, and care of animals.
- 13. B. All newly received animals from outside sources shall be isolated and their health status shall be evaluated in accordance with acceptable veterinary medical practice. C. At the initiation of a nonclinical laboratory study, animals shall be free of any disease or condition that might interfere with the purpose or conduct of the study. If, during the course of the study, the animals contract such a disease or condition, the diseased animals shall be isolated, if necessary. These animals may be treated for disease or signs of disease provided that such treatment does not interfere with the study. The diagnosis, authorizations of treatment, description of treatment, and each date of treatment shall be documented and shall be retained. D. Warm-blooded animals, excluding suckling rodents, used in laboratory procedures that require manipulations and observations over an extended period of time or in studies that require the animals to be removed from and returned to their home cages for any reason (e.g., cage cleaning, treatment, etc.), shall receive appropriate identification. All information needed to specifically identify each animal within an animal-housing unit shall appear on the outside of that unit. E. Animals of different species shall be housed in separate rooms when necessary. Animals of the same species, but used in different studies, should not ordinarily be housed in the same room when inadvertent exposure to control or test articles or animal mixup could affect the outcome of either study. If such mixed housing is necessary, adequate differentiation by space and identification shall be made. F. Animal cages, racks and accessory equipment shall be cleaned and sanitized at appropriate intervals. G. Feed and water used for the animals shall be analyzed periodically to ensure that contaminants known to be capable of interfering with the study and reasonably expected to be present in such feed or water are not present at levels above those specified in the protocol. Documentation of such analyses shall be maintained as raw data. H. Bedding used in animal cages or pens shall not interfere with the purpose or conduct of the study and shall be changed as often as necessary to keep the animals dry and clean. I. If any pest control materials are used, the use shall be documented. Cleaning and pest control materials that interfere with the study shall not be used. Subpart F - Test and Control Articles Test and control article characterization A. The identity, strength, purity, and composition or other characteristics which will appropriately define the test or control article shall be determined for each batch and shall be documented. Methods of synthesis, fabrication, or derivation of the test and control articles shall be documented by the sponsor or the testing facility. In those cases where marketed products are used as control articles, such products will be characterized by their labeling. B. The stability of each test or control article shall be determined by the testing facility or by the sponsor either:
- 14. 1. Before study initiation, or 2. Concomitantly according to written standard operating procedures, which provide for periodic analysis of each batch. C. Each storage container for a test or control article shall be labeled by name, chemical abstract number or code number, batch number, expiration date, if any, and, where appropriate, storage conditions necessary to maintain the identity, strength, purity, and composition of the test or control article. Storage containers shall be assigned to a particular test article for the duration of the study. D. For studies of more than 4 weeks' duration, reserve samples from each batch of test and control articles shall be retained for the period of time provided by §58.195. Test and control article handling Procedures shall be established for a system for the handling of the test and control articles to ensure that: a. There is proper storage. b. Distribution is made in a manner designed to preclude the possibility of contamination, deterioration, or damage. c. Proper identification is maintained throughout the distribution process. d. The receipt and distribution of each batch is documented. Such documentation shall include the date and quantity of each batch distributed or returned. Mixtures of articles with carriers A. For each test or control article that is mixed with a carrier, tests by appropriate analytical methods shall be conducted: 1. To determine the uniformity of the mixture and to determine, periodically, the concentration of the test or control article in the mixture. 2. To determine the stability of the test and control articles in the mixture as required by the conditions of the study either: a. Before study initiation, or b. Concomitantly according to written standard operating procedures which provide for periodic analysis of the test and control articles in the mixture. B. [Reserved]. C. Where any of the components of the test or control article carrier mixture has an expiration date, that date shall be clearly shown on the container. If more than one component has an expiration date, the earliest date shall be show. Subpart G - Protocol for and Conduct of a Nonclinical Laboratory Study Protocol A. Each study shall have an approved written protocol that clearly indicates the objectives and all methods for the conduct of the study. The protocol shall contain, as applicable, the following information: 1. A descriptive title and statement of the purpose of the study. 2. Identification of the test and control articles by name, chemical abstract number, or code number.
- 15. 3. The name of the sponsor and the name and address of the testing facility at which the study is being conducted. 4. The number, body weight range, sex, source of supply, species, strain, substrain, and age of the test system. 5. The procedure for identification of the test system. 6. A description of the experimental design, including the methods for the control of bias. 7. A description and/or identification of the diet used in the study as well as solvents, emulsifiers, and/or other materials used to solubilize or suspend the test or control articles before mixing with the carrier. The description shall include specifications for acceptable levels of contaminants that are reasonably expected to be present in the dietary materials and are known to be capable of interfering with the purpose or conduct of the study if present at levels greater than established by the specifications. 8. Each dosage level, expressed in milligrams per kilogram of body weight or other appropriate units, of the test or control article to be administered and the method and frequency of administration. 9. The type and frequency of tests, analyses, and measurements to be made. 10. The records to be maintained. 11. The date of approval of the protocol by the sponsor and the dated signature of the study director. 12. A statement of the proposed statistical methods to be used. B. All changes in or revisions of an approved protocol and the reasons therefore shall be documented, signed by the study director, dated, and maintained with the protocol. Conduct of a nonclinical laboratory study A. The nonclinical laboratory study shall be conducted in accordance with the protocol. B. The test systems shall be monitored in conformity with the protocol. C. Specimens shall be identified by test system, study, nature, and date of collection. This information shall be located on the specimen container or shall accompany the specimen in a manner that precludes error in the recording and storage of data. D. Records of gross findings for a specimen from postmortem observations should be available to a pathologist when examining that specimen histo pathologically. E. All data generated during the conduct of a nonclinical laboratory study, except those that are generated by automated data collection systems, shall be recorded directly, promptly, and legibly in ink. All data entries shall be dated on the date of entry and signed or initialed by the person entering the data. Any change in entries shall be made so as not to obscure the original entry, shall indicate the reason for such change, and shall be dated and signed or identified at the time of the change. In automated data collection systems, the individual responsible for direct data input shall be identified at the time of data input. Any change in automated data entries shall be made so as not to obscure the original
- 16. entry, shall indicate the reason for change, shall be dated, and the responsible individual shall be identified. Subparts H - I [Reserved] Subpart J - Records and Reports Reporting of nonclinical laboratory study results A. A final report shall be prepared for each nonclinical laboratory study and shall include, but not necessarily be limited to, the following: 1. Name and address of the facility performing the study and the dates on which the study was initiated and completed. 2. Objectives and procedures stated in the approved protocol, including any changes in the original protocol. 3. Statistical methods employed for analyzing the data. 4. The test and control articles identified by name, chemical abstracts number or code number, strength, purity, and composition or other appropriate characteristics. 5. Stability of the test and control articles under the conditions of administration. 6. A description of the methods used. 7. A description of the test system used. Where applicable, the final report shall include the number of animals used, sex, body weight range, source of supply, species, strain and substrain, age, and procedure used for identification. 8. A description of the dosage, dosage regimen, route of administration, and duration. 9. A description of all circumstances that may have affected the quality or integrity of the data. 10. The name of the study director, the names of other scientists or professionals, and the names of all supervisory personnel, involved in the study. 11. A description of the transformations, calculations, or operations performed on the data, a summary and analysis of the data, and a statement of the conclusions drawn from the analysis. 12. The signed and dated reports of each of the individual scientists or other professionals involved in the study. 13. The locations where all specimens, raw data, and the final report are to be stored. 14. The statement prepared and signed by the quality assurance unit as described in §58.35(b)(7). B. The final report shall be signed and dated by the study director. C. Corrections or additions to a final report shall be in the form of an amendment by the study director. The amendment shall clearly identify that part of the final report that is being added to or corrected and the reasons for the correction or addition, and shall be signed and dated by the person responsible storage and retrieval of records and data. a. All raw data, documentation, protocols, final reports, and specimens (except those specimens obtained from mutagenicity tests and wet
- 17. specimens of blood, urine, feces, and biological fluids) generated as a result of a nonclinical laboratory study shall be retained. b. There shall be archives for orderly storage and expedient retrieval of all raw data, documentation, protocols, specimens, and interim and final reports. Conditions of storage shall minimize deterioration of the documents or specimens in accordance with the requirements for the time period of their retention and the nature of the documents or specimens. A testing facility may contract with commercial archives to provide a repository for all material to be retained. Raw data and specimens may be retained elsewhere provided that the archives have specific reference to those other location. c. An individual shall be identified as responsible for the archives. d. Only authorized personnel shall enter the archives. e. Material retained or referred to in the archives shall be indexed to permit expedient retrieval. Retention of records A. Record retention requirements set forth in this section do not supersede the record retention requirements of any other regulations in this chapter. B. Except as provided in paragraph C of this section, documentation records, raw data and specimens pertaining to a nonclinical laboratory study and required to be made by this part shall be retained in the archive(s) for whichever of the following periods is shortest: 1. A period of at least 2 years following the date on which an application for a research or marketing permit, in support of which the results of the nonclinical laboratory study were submitted, is approved by the Food and Drug Administration. This requirement does not apply to studies supporting investigational new drug applications (IND's) or applications for investigational device exemptions (IDE's), records of which shall be governed by the provisions of paragraph A (2) of this section. 2. A period of at least 5 years following the date on which the results of the nonclinical laboratory study are submitted to the Food and Drug Administration in support of an application for a research or marketing permit. 3. In other situations (e.g., where the nonclinical laboratory study does not result in the submission of the study in support of an application for a research or marketing permit), a period of at least 2 years following the date on which the study is completed, terminated, or discontinued. C. Wet specimens (except those specimens obtained from mutagenicity tests and wet specimens of blood, urine, feces, and biological fluids), samples of test or control articles, and specially prepared material, which are relatively fragile and differ markedly in stability and quality during storage, shall be retained only as long as the quality of the preparation affords evaluation. In no case shall retention be required for longer periods than those set forth in paragraphs A and B of this section. D. The master schedule sheet, copies of protocols, and records of quality assurance inspections, as required by §58.35(c) shall be maintained by the quality assurance unit as an easily accessible system of records for
- 18. the period of time specified in paragraphs A and B of this section. E. Summaries of training and experience and job descriptions required to be maintained by §58.29 B may be retained along with all other testing facility employment records for the length of time specified in paragraphs A and B of this section. F. Records and reports of the maintenance and calibration and inspection of equipment, as required by §58.63 B and C, shall be retained for the length of time specified in paragraph B of this section. G. Records required by this part may be retained either as original records or as true copies such as photocopies, microfilm, microfiche, or other accurate reproductions of the original records. H. If a facility conducting nonclinical testing goes out of business, all raw data, documentation, and other material specified in this section shall be transferred to the archives of the sponsor of the study. The Food and Drug Administration shall be notified in writing of such a transfer. Subpart K - Disqualification of Testing Facilities Purpose A. The purposes of disqualification are: 1. To permit the exclusion from consideration of completed studies that were conducted by a testing facility which has failed to comply with the requirements of the good laboratory practice regulations until it can be adequately demonstrated that such noncompliance did not occur during, or did not affect the validity or acceptability of data generated by, a particular study; and 2. To exclude from consideration all studies completed after the date of disqualification until the facility can satisfy the Commissioner that it will conduct studies in compliance with such regulations. a. The determination that a nonclinical laboratory study may not be considered in support of an application for a research or marketing permit does not, however, relieve the applicant for such a permit of any obligation under any other applicable regulation to submit the results of the study to the Food and Drug Administration. Grounds for disqualification The Commissioner may disqualify a testing facility upon finding all of the following: A. The testing facility failed to comply with one or more of the regulations set forth in this part (or any other regulations regarding such facilities in this chapter); B. The noncompliance adversely affected the validity of the nonclinical laboratory studies; and C. Other lesser regulatory actions (e.g., warnings or rejection of individual studies) have not been or will probably not be adequate to achieve compliance with the good laboratory practice regulations. Notice of and opportunity for hearing on proposed disqualification A. Whenever the Commissioner has information indicating that grounds exist under §58.202 which in his opinion justify disqualification of a
- 19. testing facility, he may issue to the testing facility a written notice proposing that the facility be disqualified. B. A hearing on the disqualification shall be conducted in accordance with the requirements for a regulatory hearing set forth in part 16 of this chapter. Final order on disqualification A. If the Commissioner, after the regulatory hearing, or after the time for requesting a hearing expires without a request being made, upon an evaluation of the administrative record of the disqualification proceeding, makes the findings required in §58.202, he shall issue a final order disqualifying the facility. Such order shall include a statement of the basis for that determination. Upon issuing a final order, the Commissioner shall notify (with a copy of the order) the testing facility of the action. B. If the Commissioner, after a regulatory hearing or after the time for requesting a hearing expires without a request being made, upon an evaluation of the administrative record of the disqualification proceeding, does not make the findings required in §58.202, he shall issue a final order terminating the disqualification proceeding. Such order shall include a statement of the basis for that determination. Upon issuing a final order the Commissioner shall notify the testing facility and provide a copy of the order. Actions upon disqualification A. Once a testing facility has been disqualified, each application for a research or marketing permit, whether approved or not, containing or relying upon any nonclinical laboratory study conducted by the disqualified testing facility may be examined to determine whether such study was or would be essential to a decision. If it is determined that a study was or would be essential, the Food and Drug Administration shall also determine whether the study is acceptable, notwithstanding the disqualification of the facility. Any study done by a testing facility before or after disqualification may be presumed to be unacceptable, and the person relying on the study may be required to establish that the study was not affected by the circumstances that led to the disqualification, e.g., by submitting validating information. If the study is then determined to be unacceptable, such data will be eliminated from consideration in support of the application; and such elimination may serve as new information justifying the termination or withdrawal of approval of the application. B. No nonclinical laboratory study begun by a testing facility after the date of the facility's disqualification shall be considered in support of any application for a research or marketing permit, unless the facility has
- 20. been reinstated under§58.219. The determination that a study may not be considered in support of an application for a research or marketing permit does not, however, relieve the applicant for such a permit of any obligation under any other applicable regulation to submit the results of the study to the Food and Drug Administration. Public disclosure of information regarding disqualification A. Upon issuance of a final order disqualifying a testing facility under §58.206(a), the Commissioner may notify all or any interested persons. Such notice may be given at the discretion of the Commissioner whenever he believes that such disclosure would further the public interest or would promote compliance with the good laboratory practice regulations set forth in this part. Such notice, if given, shall include a copy of the final order issued under §58.206(a) and shall state that the disqualification constitutes a determination by the Food and Drug Administration that nonclinical laboratory studies performed by the facility will not be considered by the Food and Drug Administration in support of any application for a research or marketing permit. If such notice is sent to another Federal Government agency, the Food and Drug Administration will recommend that the agency also consider whether or not it should accept nonclinical laboratory studies performed by the testing facility. If such notice is sent to any other person, it shall state that it is given because of the relationship between the testing facility and the person being notified and that the Food and Drug Administration is not advising or recommending that any action be taken by the person notified. B. A determination that a testing facility has been disqualified and the administrative record regarding such determination are disclosable to the public under part 20 of this chapter. Alternative or additional actions to disqualification A. Disqualification of a testing facility under this subpart is independent of, and neither in lieu of nor a precondition to, other proceedings or actions authorized by the act. The Food and Drug Administration may, at any time, institute against a testing facility and/or against the sponsor of a nonclinical laboratory study that has been submitted to the Food and Drug Administration any appropriate judicial proceedings (civil or criminal) and any other appropriate regulatory action, in addition to or in lieu of, and prior to, simultaneously with, or subsequent to, disqualification. The Food and Drug Administration may also refer the matter to another Federal, State, or local government law enforcement or regulatory agency for such action as that agency deems appropriate. B. The Food and Drug Administration may refuse to consider any particular nonclinical laboratory study in support of an application for a research or marketing permit, if it finds that the study was not
- 21. conducted in accordance with the good laboratory practice regulations set forth in this part, without disqualifying the testing facility that conducted the study or undertaking other regulatory action. Suspension or termination of a testing facility by a sponsor Termination of a testing facility by a sponsor is independent of, and neither in lieu of nor a precondition to, proceedings or actions authorized by this subpart. If a sponsor terminates or suspends a testing facility from further participation in a nonclinical laboratory study that is being conducted as part of any application for a research or marketing permit that has been submitted to any Center of the Food and Drug Administration (whether approved or not), it shall notify that Center in writing within 15 working days of the action; the notice shall include a statement of the reasons for such action. Suspension or termination of a testing facility by a sponsor does not relieve it of any obligation under any other applicable regulation to submit the results of the study to the Food and Drug Administration. Reinstatement of a disqualified testing facility A testing facility that has been disqualified may be reinstated as an acceptable source of nonclinical laboratory studies to be submitted to the Food and Drug Administration if the Commissioner determines, upon an evaluation of the submission of the testing facility, that the facility can adequately assure that it will conduct future nonclinical laboratory studies in compliance with the good laboratory practice regulations set forth in this part and, if any studies are currently being conducted, that the quality and integrity of such studies have not been seriously compromised. A disqualified testing facility that wishes to be so reinstated shall present in writing to the Commissioner reasons why it believes it should be reinstated and a detailed description of the corrective actions it has taken or intends to take to assure that the acts or omissions which led to its disqualification will not recur. The Commissioner may condition reinstatement upon the testing facility being found in compliance with the good laboratory practice regulations upon an inspection. If a testing facility is reinstated, the Commissioner shall so notify the testing facility and all organizations and persons who were notified, under §58.213 of the disqualification of the testing facility. A determination that a testing facility has been reinstated is disclosable to the public under part 20 of this chapter. Good manufacturing practices Introduction: This document (Guide) is intended to provide guidance regarding good manufacturing practice (GMP) for the manufacturing of active pharmaceutical ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the requirements for quality and purity that they purport or are represented to possess. In this Guide “manufacturing” is defined to include all operations
- 22. of receipt of materials, production, packaging, repackaging, labelling, relabeling, quality control, release, storage and distribution of APIs and the related controls. In this Guide the term “should” indicates recommendations that are expected to apply unless shown to be inapplicable or replaced by an alternative demonstrated to provide at least an equivalent level of quality assurance. For the purposes of this Guide, the terms “current good manufacturing practices” and “good manufacturing practices” are equivalent. Scope: This Guide applies to the manufacture of APIs for use in human drug (medicinal) products. It applies to the manufacture of sterile APIs only up to the point immediately prior to the APIs being rendered sterile. The sterilization and aseptic processing of sterile APIs are not covered by this guidance, but should be performed in accordance with GMP guidelines for drug (medicinal) products as defined by local authorities. This Guide covers APIs that are manufactured by chemical synthesis, extraction, cell culture/fermentation, by recovery from natural sources, or by any combination of these processes. Specific guidance for APIs manufactured by cell culture/fermentation is described in Section 18. This Guide excludes all vaccines, whole cells, whole blood and plasma, blood and plasma derivatives (plasma fractionation), and gene therapy APIs. An “API Starting Material” is a raw material, intermediate, or an API that is used in the production of an API and that is incorporated as a significant structural fragment into the structure of the API. An API Starting Material can be an article of commerce, a material purchased from one or more suppliers under contract or commercial agreement, or produced in-house. API Starting Materials normally have defined chemical properties and structure. Quality management Principles: Quality should be the responsibility of all persons involved in manufacturing. Each manufacturer should establish, document, and implement an effective system for managing quality that involves the active participation of management and appropriate manufacturing personnel. The system for managing quality should encompass the organizational structure, procedures, processes and resources, as well as activities necessary to ensure confidence that the API will meet its intended specifications for quality and purity. All quality related activities should be defined and documented. There should be a quality unit(s) that is independent of production and that fulfills both quality assurance (QA) and quality control (QC) responsibilities. This can be in the form of separate QA and QC units or a single individual or group, depending upon the size and structure of the organization.
- 23. The persons authorized to release intermediates and APIs should be specified. All quality related activities should be recorded at the time they are performed. Any deviation from established procedures should be documented and explained. Critical deviations should be investigated, and the investigation and its conclusions should be documented. No materials should be released or used before the satisfactory completion of evaluation by the quality unit(s) unless there are appropriate systems in place to allow for such use (e.g. release under quarantine as described in Section 10.20 or the use of raw materials or intermediates pending completion of evaluation). Procedures should exist for notifying responsible management in a timely manner of regulatory inspections, serious GMP deficiencies, product defects and related actions (e.g., quality related complaints, recalls, regulatory actions, etc.). Responsibilities of the Quality Unit(s) The quality unit(s) should be involved in all quality-related matters. The quality unit(s) should review and approve all appropriate quality- related documents. The main responsibilities of the independent quality unit(s) should not be delegated. These responsibilities should be described in writing and should include but not necessarily be limited to: 1. Releasing or rejecting all APIs. Releasing or rejecting intermediates for use outside the control of the manufacturing company; 2. Establishing a system to release or reject raw materials, intermediates, packaging and labelling materials; 3. Reviewing completed batch production and laboratory control records of critical process steps before release of the API for distribution; 4. Making sure that critical deviations are investigated and resolved; 5. Approving all specifications andmaster production instructions; 6. Approving all procedures impacting the quality of intermediates or APIs; 7. Making sure that internal audits (self-inspections) are performed; 8. Approving intermediate and API contract manufacturers; 9. Approving changes that potentially impact intermediate or API quality; 10. Reviewing and approving validation protocols and reports; 11. Making sure that quality related complaints are investigated and resolved; 12. Making sure that effective systems are used for maintaining and calibrating critical equipment; 13. Making sure that materials are appropriately tested and the results are reported; 14. Making sure that there is stability data to support retest or expiry dates and storage conditions on APIs and/or intermediates where
- 24. appropriate; and 15. Performing product quality reviews (as defined in Section 2.5). Responsibility for Production Activities The responsibility for production activities should be described in writing, and should include but not necessarily be limited to: 1. Preparing, reviewing, approving and distributing the instructions for the production of intermediates or APIs according to written procedures; 2. Producing APIs and, when appropriate, intermediates according to preapproved instructions; 3. Reviewing all production batch records and ensuring that these are completed and signed; 4. Making sure that all production deviations are reported and evaluated and that critical deviations are investigated and the conclusions are recorded; 5. Making sure that production facilities are clean and when appropriate disinfected 6. Making sure that the necessary calibrations are performed and records kept; 7. Making sure that the premises and equipment are maintained and records kept; 8. Making sure that validation protocols and reports are reviewed and approved; 9. Evaluating proposed changes in product, process or equipment; and 10. Making sure that new and, when appropriate, modified facilities and equipment are qualified. Internal Audits (Self Inspection) In order to verify compliance with the principles of GMP for APIs, regular internal audits should be performed in accordance with an approved schedule. Audit findings and corrective actions should be documented and brought to the attention of responsible management of the firm. Agreed corrective actions should be completed in a timely and effective manner Product Quality Review Regular quality reviews of APIs should be conducted with the objective of verifying the consistency of the process. Such reviews should normally be conducted and documented annually and should include at least: A review of critical in-process control and critical API test results A review of all batches that failed to meet established specification(s); A review of all critical deviations or non- conformances and related investigations; A review of any changes carried out to the processes or analytical methods; A review of results of the stability monitoring program; A review of all quality-related returns, complaints and recalls; and
- 25. A review of adequacy of corrective actions. Personnel: There should be an adequate number of personnel qualified by appropriate education, training and/or experience to perform and supervise the manufacture of intermediates and APIs. The responsibilities of all personnel engaged in the manufacture of intermediates and APIs should be specified in writing Training should be regularly conducted by qualified individuals and should cover, at a minimum, the particular operations that the employee performs and GMP as it relates to the employee's functions. Records of training should be maintained. Training should be periodically assessed. Personnel Hygiene Personnel should practice good sanitation and health habits Personnel should wear clean clothing suitable for the manufacturing activity with which they are involved and this clothing should be changed when appropriate. Additional protective apparel, such as head, face, hand, and arm coverings, should be worn when necessary, to protect intermediates and APIs from contamination. Personnel should avoid direct contact with intermediates or APIs. Smoking, eating, drinking, chewing and the storage of food should be restricted to certain designated areas separate from the manufacturing areas. Personnel suffering from an infectious disease or having open lesions on the exposed surface of the body should not engage in activities that could result in compromising the quality of APIs. Any person shown at any time (either by medical examination or supervisory observation) to have an apparent illness or open lesions should be excluded from activities where the health condition could adversely affect the quality of the APIs until the condition is corrected or qualified medical personnel determine that the person's inclusion would not jeopardize the safety or quality of the APIs. Building and facilities Design and Construction: Buildings and facilities used in the manufacture of intermediates and APIs should be located, designed, and constructed to facilitate cleaning, maintenance, and operations as appropriate to the type and stageof manufacture. Facilities should also be designed to minimize potential contamination. Where microbiological specifications have been established for the intermediate or API, facilities should also be designed to limit exposure to objectionable microbiological contaminants as appropriate Buildings and facilities should have adequate space for the orderly placement of equipment and materials to prevent mix-ups and contamination. Where the equipment itself (e.g., closed or contained systems) provides adequate protection of the material, such equipment can be located outdoors.
- 26. The flow of materials and personnel through the building or facilities should be designed to prevent mix-ups or contamination. There should be defined areas or other control systems for the following activities: Receipt, identification, sampling, and quarantine of incoming materials, pending release or rejection; Quarantine before release or rejection of intermediates and APIs; Sampling of intermediates and APIs Holding rejected materials before further disposition (e.g., return, reprocessing or destruction); Storage of released materials; Production operations; Packaging and labelling operations; and Laboratory operations.
- 27. 1 cGMP GUIDELINES ACCORDING TO SCHEDULE M, USFDA (INCLUSIVE OF CDER AND CBER) PHARMACEUTICAL INSPECTION CONVENTION (PIC), WHO AND EMEA GMP GUIDELINES AS PER SCHEDULE M GMP: • GMP is that part of Quality assurance which ensures that the products are consistently manufactured and controlled to the Quality standards appropriate to their intended use. • "GMP" - A set of principles and procedures which, when followed by manufacturers for therapeutic goods, helps ensure that the products manufactured will have the required quality. CGMP : • Usually see “cGMP” – where c = current, to emphasize that the expectations are dynamic . GMP COVERS.. • GMPs ➢ Organisation and Personnel ➢ Buildings and Facilities ➢ Equipment ➢ Control of Components, Containers and Closures ➢ Production and Process control ➢ Holding and Distribution ➢ Laboratory Controls ➢ Records and Reports ➢ Returned and salvaged products • All aspects of production; from the starting materials, premises and equipment to the training and personal hygiene of staff . • Detailed, written procedures are essential for each process that could affect the quality of the finished product. • There must be systems to provide documented proof that correct procedures are consistently followed at each step in the manufacturing process - every time a product is made.
- 28. 2 DRUG AND COSMETIC ACT IN INDIA • In 1937 a Bill was introduced in the Central Legislative Assembly to give effect to the recommendations of the Drugs Enquiry Committee to regulate the import of drugs into British India. • Provincial Governments got the resolution passed from the Provincial Legislatures and sent them to the Central Government for getting through the Bill to regulate the import, manufacture, distribution and sale of Drugs and Cosmetics. There upon the Drugs and Cosmetics Bill was introduced in the Central Legislative Assembly. • The Drugs and Cosmetics Bill was passed by the Central Legislative Assembly and it received the assent of the Governor General on 10th April, 1940 and thus became the Drugs and Cosmetics Act, 1940 (23 of 1940). • An Act to regulate the import, manufacture, distribution and sale of drugs [(Note: Ins. by Act 21 of 1962, sec.2 (w.e.f. 27-7-1964)) and cosmetics]. DRUG AND COSMETIC ACT 1940 SCHEDULE M 1. General Requirements : a) Location and surroundings- The factory building(s) for manufacture of drugs shall be so situated that it avoid risk of contamination from external environmental including open sewage, drain, public lavatory. b) Building and premises- They shall conform to the conditions laid down in the Factories Act, 1948 (63 of 1948) The premises used for manufacturing, processing, warehousing, packaging labelling and testing purposes shall be: i. compatible with other drug manufacturing operations. ii. adequately working space to avoid the risk of mix-up between different (b) avoid the possibilities of contamination and cross contamination. iii. designed / constructed / maintained to prevent entry of insects, pests, birds, and rodents. iv. well lighted, effectively ventilated, with proper Air Handling Units. c) Water System-
- 29. 3 ➢ There shall be validated system for treatment of water in accordance with standards specified by the Bureau of Indian Standards or Local Municipality or Pharmacopoeial specification. ➢ Water shall be stored in tanks, which do not adversely affect quality of water and ensure freedom from microbiological growth. ➢ The tank shall be cleaned periodically and records maintained by the licensee in this behalf. d) Disposal of Waste- i. The disposal of sewage and effluents (solid, liquid and gas) be in conformity with the requirements of Environment Pollution Control Board. ii. All bio-medical waste shall be destroyed as per the provisions of the Bio-Medical Waste (Management and Handling) Rules, 1996. iii. Records shall be maintained for all disposal of waste. iv. Provisions shall be made for the proper and safe storage of waste materials awaiting disposal. 2. Warehousing Area : • Adequate areas to allow orderly warehousing of various categories of materials. • Good storage conditions. • There shall be a separate sampling area in the warehousing area for active raw materials and excipients. • Segregation shall be provided for the storage of rejected, recalled or returned materials or products. • Highly hazardous, poisonous and explosive materials shall be stored in safe and secure areas. 3. Production Area : • Separate dedicated and self-contained facilities shall be made available for the production of sensitive pharmaceutical products. • Orderly and logical positioning of equipment and materials and movement of personnel and to minimize risk of omission or wrong application of any manufacturing and control measures. • Services lines shall preferably be identified by colours and the nature of the supply and direction of the flow shall be marked/indicated.
- 30. 4 4. Ancillary Areas : • Ancillary Areas 4.1 Rest and refreshment rooms shall be separate from other areas. Facilities for changing, storing clothes and for washing and toilet purposes shall be adequate for the number of users. • Animal houses shall be those as prescribed in Rule 150-C(3) of the Drugs and Cosmetics Rules, 1945 which shall be adopted for production purposes. 5. Quality Control Area : • QC Lab. shall be independent of the production areas. Separate areas each for physico-chemical, biological, microbiological or radio-isotope analysis. • Adequate space shall be provided to avoid mix-ups and proper storage for test samples, retained samples, reference standards, reagents and records. 6. Personnel : • Qualifications and practical experience in the relevant field. • Written duties of technical and Quality Control personnel shall be laid and following strictly. • Number of personnel employed shall be adequate and in direct proportion to the workload. 7. Health, Clothing and Sanitation of Workers : • Prior to employment, all personnel, shall undergo medical examination and a periodically examination is carried out, records are also maintained. • Proper training shall be given to all employees to maintain personnel hygiene and adequate facilities for personal cleanliness. • Smoking, eating, drinking, chewing , food, drink and personal medicines not permitted in production, laboratory, storage area. 8. Manufacturing Operations and Controls : • All manufacturing operations shall be carried out under the supervision of technical staff approved by the Licensing Authority. • Precautions against mix-up and cross-contamination-
- 31. 5 i. By proper air handling system, pressure differential, segregation, status labelling and cleaning. Proper records and SOP there of shall be maintained. ii. Processing of sensitive drugs and cytotoxic substances in segregated areas. iii. Proper labelling of materials and equipments. iv. Packaging lines shall be independent and adequately segregated. v. All printing and overprinting shall be authorized in writing. vi. The manufacturing environment maintained at the required levels of temperature, humidity and cleanliness. 9. Sanitation in the Manufacturing Premises : • The manufacturing premises shall be cleaned and in an orderly manner. • The manufacturing areas shall not be used for other operations. • A routine sanitation program shall be drawn up which shall be properly recorded and which indicate- i. specific areas to be cleaned and cleaning intervals. ii. cleaning procedure to be followed. iii. personnel assigned to and responsible for the cleaning operation. 10. Raw Materials : • Keeping an inventory of all raw materials to be used and maintain records as per Schedule U. • Authorized staff who examine each consignment for its integrity. • Labelling with the following information: i. name of the product and the internal code reference and analytical reference number. ii. manufacturer’s name, address and batch number. iii. the status of the contents (e.g. quarantine, under test, released, approved, rejected); and iv. the manufacturing date, expiry date and re-test date. 11. Equipment : • Equipment shall be located, designed, constructed, adapted to suit the operations and logbook is maintained.
- 32. 6 • Equipment shall be calibrated and checked on a scheduled basis in accordance to SOP and maintain records. • Equipment shall be inert and defective are removed and labelled. 12. Documentation and Records : Documentation and Records. Documentation is an essential part of the Quality assurance system and Its aim is to define the specifications for all materials, method of manufacture and control to release a batch of drug for sale. • Documents shall be approved, signed and dated by authorized persons. • Documents shall specify : the title, nature and purpose laid out in an orderly fashion kept up to date. • SOP shall be retained for at least one year after the expiry date of the finished product. • Data may be recorded by electronic data processing systems but Master Formulae and detailed operating procedures relating to the system in use shall also be available in a hard copy to facilitate checking of the accuracy of the records. 13. Labels and Other Printed Materials : Labels are necessary for identification of the drugs and their use. The Printing shall be done in bright colours and in a legible manner. The label shall carry all the prescribed details about the product. • All containers and equipment shall bear appropriate labels. • Prior to release, all labels for containers shall be examined by the QC Department. • Records of receipt of all labelling and packaging materials shall be maintained and unused coded and damaged labels and packaging materials shall be destroyed and recorded. 14. Quality Assurance : It is a wide-ranging concept concerning all matters that individually or collectively influence the quality of a product. It shall ensure that: • the pharmaceutical products are designed and developed in a way that takes account of the requirement of GMP ,GLP and GCP. • adequate controls on starting materials, intermediate products, and other in-process controls, calibrations, and validations are carried out. • products are released after authorized persons have certified.
- 33. 7 15. Self-inspection and Quality Audit : It is for the assessment of all or part of a system with the specific purpose of improving it. • The program is designed to detect shortcomings in the implementation of GMP and to recommend the necessary corrective actions. Self-inspections shall be performed routinely and on specific occasions. The team responsible for self-inspection shall consist of independent, experienced, qualified persons from within or outside the company who can evaluate the implementation of GMP objectively; all recommendations for corrective action shall be implemented. • The procedure for self-inspection shall be documented indicating self- inspection results; evaluation, conclusions and recommended corrective actions with effective follow up program. 16. Quality Control System : Quality control shall be concerned with sampling, specifications, testing, documentation, release procedures. Materials are not released for use, nor products released for sale or supply until their quality has been judged to be satisfactory. • QC lab should have qualified and experience staff. • QC lab. may be divided into Chemical, Instrumentation, Microbiological and Biological testing. • The QC department shall conduct stability studies of the products to ensure and assign their shell life . All records of such studies shall be maintained. • All instruments shall be calibrated and validated before adopted for routine testing. • Pharmacopoeia, reference standards, working standards, references spectra, other reference materials and technical books, as required, shall be available in the QC Laboratory. 17. Specification : • For raw materials and packaging materials. They shall include: i. the designated name and internal code reference. ii. reference, if any, to a pharmacopoeial monograph. iii. qualitative and quantitative requirements with acceptance limits. iv. name and address of manufacturer or supplier and original manufacturer of the material.
- 34. 8 v. specimen of printed material. vi. directions for sampling and testing or reference to procedures. vii. storage conditions and viii. maximum period of storage before re-testing. • For Finished Products: Appropriate specifications for finished products shall include: - i. the designated name of the product and the code reference. ii. the formula or a reference to the formula and the pharmacopoeial reference. iii. directions for sampling and testing or a reference to procedures. iv. a description of the dosage form and package details. v. the storage conditions and precautions, where applicable. vi. the shelf-life. 18. Master Formula Records : There shall be Master Formula records relating to all manufacturing procedures for each product and batch size. These shall be prepared and endorsed by head of production and quality control. The Master Formula shall include: • the name of the product together with product reference code relating to its specifications. • the patent or proprietary name of the product along with the generic name, a description of the dosage form, strength, composition of the product and batch size. • name, quantity, and reference number of all the starting materials to be used. • A statement of the processing location and the principal equipment to be used. • detailed stepwise processing instructions and the time taken for each step. • the instructions for in-process control with their limits. • the requirements for storage conditions of the products, including the container, labelling and special storage conditions where applicable. • any special precautions to be observed; and • packing details and specimen labels. 19. Packing Records : There shall be authorised packaging instructions for each product, pack size and type . These shall include or have a reference to the following: -
- 35. 9 • name of the product. • description of the dosage form, strength and composition. • the pack size expressed in terms of the number of doses, weight or volume of the product in the final container. • special precautions to be observed, including a careful examination of the area and equipment in order to ascertain the line clearance before the operations begin. 20. Batch Processing Records : A batch packaging record shall be kept for each batch or part batch processed. It shall be based on the relevant parts of the packaging instructions, and the method of preparation of such records shall be designed to avoid transcription errors. 21. Batch Processing Records: There shall be Batch Processing Record for each product. It shall be based on the relevant parts of the currently approved Master Formula. The method of preparation of such records included in the Master Formula shall be designed to avoid transcription errors. 22. Standard Operating Procedures (SOPs) : There shall be written SOP and records for the: • Receipt of each delivery of raw, primary and printed material. • Internal labelling, quarantine and storage of starting materials, packaging materials and other materials, as appropriate. • For each instrument and equipment. • Sampling which include the person(s) authorized to take the samples. • Describing the details of the batch numbering which ensure that each batch of intermediate, bulk or finished product is identified with a specific batch number. • Testing materials and products at different stages of manufacture, describing the methods and equipment to be used. 23. Reference Samples : • Each lot of every active ingredient, in a quality sufficient to carryout all the tests, except sterility and pyrogens / Bacterial Endotoxin Test, shall be retained for a period of 3 months after the date of expiry of the last batch produced from that active ingredient.
- 36. 10 • Samples of finished formulations shall be stored in the same or simulated containers in which the drug has been actually marketed. 24. Reprocessing and Recoveries : • When reprocessing is necessary, written procedures shall be established and approved by quality assurance department. • Recovery of product residue may be carried out, if permitted, in the master production. 25. Distribution Records : Records of distribution shall be maintained in a manner such that finished batch of a drug can be traced to the retain level to facilitate prompt and complete recall of the batch, if and when necessary. 26. Validation and Process Validation : • Validation studies shall be an essential part of GMP and shall be conducted as per the pre-defined protocols. • A written report summarizing recorded results and conclusions shall be prepared, documented and maintained. • When any new Master Formula or method of preparation is adopted, steps shall be taken to demonstrate its suitability for routine processing. • Significant changes to the manufacturing process, including any changes in equipment or materials shall be validated. 27. Product Recalls : • A prompt and effective product recall system of defective products shall be devised for timely information of al concerned stockist, wholesalers, suppliers, up to the retail level within the shortest period. • The license may make use of both print and electronic media in this regard. 28. Complaints and Adverse Reactions : • All complaints there of concerning product quality shall be carefully reviewed and recorded according to written procedures. • Each complaint shall be investigated /evaluated by the designated personnel of the company and records of investigation and remedial action taken thereof shall be maintained.
- 37. 11 • Reports of serious adverse drug reactions resulting from the use of a drug shall be forthwith reported to the concerned licensing authority. 29. Site Master File : The licensee shall prepare a succinct document in the form of Site Master File containing specific and factual GMP about the production and/or control of pharmaceutical manufacturing preparations carried out at the licensed premises. It shall contain the following: - • General information • Personnel. • Premises. • Equipment. • Sanitation. • Documentation. • Production. • Quality Control. • Loan licence manufacture and licensee. • Distribution, complaints and product recall. • Export of drugs. cGMPs According to US FDA INTRODUCTION: Good Manufacturing Practice (GMP) ensures that quality is built into the organisation and processes involved in manufacture GMP covers all aspects of “manufacture” including collection, transportation, processing, storage, quality control and delivery of the finished product. • It is designed to minimize the risks involved in any pharmaceutical production that cannot be eliminated through testing the final product Protect the integrity and quality of manufactured product intended for human use. PRINCIPLES OF cGMP: • Design and construct the facilities and equipments properly
- 38. 12 •Follow written procedures and Instructions •Document work •Validate work • Monitor facilities and equipment •Write step by step operating procedures and work on instructions •Design, develop and demonstrate job competence •Protect against contamination •Control components and product related processes •Conduct planned and periodic audits LIST OF IMPORTANT DOCUMENTS IN cGMP: •Policies •SOPs •Specifications •MFR (Master Formula Record) •Batch Package Records (BMR) •Manuals •Master plans/ files •Validation protocols •Forms and Formats •Records What are CGMP?
- 39. 13 • c GMPs cover a broad range of methods, practices and principles that are implemented and documented during product development to ensure consistent manufacture of quality products •the cGMP assures the identity, strength, quality, and purity of drug products is built into the design and manufacturing process at every step. •" GMP is a dynamic concept and practice. Staying “current” is driven by technology, improved practices and regulatory issues. USFDA REGULATIONS : •The requirements for compliance to cGMP are lain down in the following code of Federal Regulation (21CFR). •21 CFR Part 210 cGMP in manufacturing, processing, packing, or holding of the drugs; General 210.1 Status of current good manufacturing practice regulations. 210.2 Applicability of current good manufacturing practice regulations. 210.3 Definitions. •21 CFR Part 211 cGMP for finished pharmaceutical. •21 CFR Part 610 – Current Good Manufacture of Biological Products •21 CFR Part 820 – Current Good Manufacturing Practices for Devices Why are cGMPs so important? •A consumer usually cannot detect that a drug product is safe. While cGMPs require testing, testing alone is not adequate to ensure quality. •FDA will often use these reports to identify sites for which an inspection or investigation is needed.
- 40. 14 Essential elements of cGMP are: •Good Documentation Practices •Training •Facilities and Equipment Management •Change Control Systems •Operations Oversight and Management CGMP for finished pharmaceuticals: part 211 •Subpart A - General Provision •Subpart B - Organization & Personnel •Subpart C - Building & Facilities •Subpart D – Equipment •Subpart E - Control of Components & Drug Product Containers & Closures •Subpart F - Production & Process Control •Subpart G - Packaging & Labeling Control •Subpart H - Handling & Distribution •Subpart I - Laboratory Control •Subpart J - Records & Reports •Subpart K - Returned & Salvaged Drugs Subpart A-General Provisions 211.1 Scope: (a) The minimum cGMP for preparation of drug products for administration to humans or animals.
- 41. 15 (b) The requirements in this part shall not be enforced for OTC drug products and all their ingredients are ordinarily marketed and consumed as human foods. 211.3 Definitions: The definitions and interpretations contained in section 201 of the act shall be applicable to such terms when used in this part and in Parts 211 Subpart B-Organization and Personnel 211.22 Responsibilities of quality control unit: (a) There shall be a QC unit that shall have the responsibility and authority to approve or reject all components, drug product containers, closures, in-process materials, packaging material, labeling . And records. (b) The responsibility for approving or rejecting all procedures or specifications impacting on the identity, strength, quality, and purity of the drug product. 211.25 Personnel qualifications: (a) Each person responsible for supervising the manufacture, processing, packing, or holding of a drug product shall have the education, training, and experience, to provide assurance that the drug product has the safety, identity, strength, quality, and purity that it is represented to possess. 211.28 Personnel responsibilities: (a) Personnel engaged in the manufacture, processing, packing, or holding of a drug product shall wear clean clothing appropriate for the duties they perform. (b) Personnel shall practice good sanitation and health habits. 211.34 Consultants: It advising on the manufacture, processing, packing, or holding of drug products shall have sufficient education, training. Records shall be maintained stating the name, address, and qualifications of any consultants and the type of service they provide. Subpart C-Buildings and Facilities
- 42. 16 211.42 Design and construction features: (a) Building shall be of suitable size, construction and location to facilitate cleaning, maintenance, and proper operations. (b) adequate space for the orderly placement of equipment and materials to prevent mix-ups between different components, drug product containers, closures, labeling , in-process materials, or drug products, and to prevent contamination. 211.44 Lighting: Adequate lighting shall be provided in all areas. 211.46 Ventilation, air filtration, air heating and cooling: (a) Adequate ventilation shall be provided. (b) Equipment for adequate control over air pressure, micro-organisms, dust, humidity, and temperature shall be provided (c) Air filtration systems, including pre-filters and particulate matter air filters, shall be used. 211.48 Plumbing: Potable water shall be supplied under continuous positive pressure in a plumbing system free of defects that could contribute contamination to any drug product. 211.50 Sewage and refuse: Sewage, trash, and other refuse in and from the building and immediate premises shall be disposed of in a safe and sanitary manner. 211.52 Washing and toilet facilities. 211.56 Sanitation. 211.58 Maintenance. 211.52-211.58 should be neat, clean and good
- 43. 17 Subpart D-Equipment 211.63 Equipment design, size, and location: Equipment shall be of appropriate design, adequate size, and suitably located to facilitate operations for its intended use and for its cleaning and maintenance. 211.65 Equipment construction: Equipment shall be constructed so that surfaces that contact components, in- process materials, or drug products shall not be reactive, additive, so as to alter the safety, identity, strength, quality, or purity of the drug product 211.67 Equipment cleaning and maintenance: •Equipment shall be cleaned, maintained, to prevent contamination that would alter the safety, identity, strength, quality, or purity of the drug product. •Inspection of equipment for cleanliness before use. •Records shall be kept of maintenance, cleaning. 211.68 Automatic, mechanical, and electronic equipment: (a) Equipments including computers, or related systems that will perform a function satisfactorily. (b) They shall be routinely calibrated, inspected, or checked according to a written program designed to assure proper performance. (c)Written records of those calibration checks and inspections shall be maintained. 211.72 Filter: (a) Fiber -releasing filters may not be used for injectable drug products unless it is not possible to manufacture such drug products without the use of such filters. (b) If use of a fiber -releasing filter is necessary, an additional non- fiber - releasing filter of 0.22 micron maximum mean porosity shall subsequently be used to reduce the content of particles in the injectable drug product. Use of an asbestos-containing filter.
- 44. 18 Subpart E-Control of Components and Drug Product Containers and Closures 211.80 General requirement: There shall be written procedures in sufficient detail the receipt, identification, storage, handling, sampling, testing, and approval or rejection of components and drug product containers and closures. 211.82 Receipt and storage of untested components, drug product containers, and closures: (a) They shall be examined visually for appropriate labeling as to contents, container damage or broken seals, and contamination. (b) They shall be stored under quarantine until they have been tested or examined, as appropriate, and released. 211.84 Testing and approval or rejection of components, drug product containers, and closures: (a) The lot has been sampled, tested, as appropriate, and released for use by the quality control unit. (b) Representative samples of each shipment of each lot shall be collected for testing or examination. 211.86 Use of approved components, drug product containers, and closures: They shall be rotated so that the oldest approved stock is used first. 211.87 Retesting of approved components, drug product containers, and closures: They shall be retested, as appropriate, for identity, strength, quality, and purity and approved or rejected by the quality control unit. Subpart F-Production and Process Controls 211.100 Written procedures; deviations: There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess. 211.101 Charge-in of components:
- 45. 19 Components for drug product manufacturing shall be weighed, measured. If a component is removed from the original container to another, the new container shall be identified with the following information: (1) Component name (2) Receiving or control number; (3) Weight or measure in new container; (4) Batch for which component was dispensed, including its product name, strength, and lot number. 211.103 Calculation of yield: Actual yields and % of theoretical yield shall be determined at manufacturing, processing, packaging, or holding of the drug product. 211.105 Equipment identification: (a) Major equipment properly identified during production. (b) Major equipment shall be identified by a distinctive identification number that shall be recorded in the batch production record to show the specific equipment used in the manufacture. 211.110 Sampling and testing of in-process materials and drug products: (a) To assure batch uniformity and integrity of drug products, (b) Written procedures shall be established that describe the in-process controls, and tests, to be conducted on appropriate samples of in-process materials of each batch. 211.111 Time limitations on production: To assure the quality of the drug product. Deviation from established time limits may be acceptable if such deviation does not compromise the quality of the drug product. 211.113 Control of microbiological contamination: Written procedures, designed to prevent microbiological contamination of drug products purporting to be sterile. Such procedures shall include validation 211.115 Reprocessing: Written procedures shall be established and followed prescribing a system for reprocessing batches that do not conform to standards or specifications and the steps to be taken to insure that the reprocessed batches will conform to all established standards, specifications, and characteristics.
- 46. 20 Subpart G-Packaging and Labeling Control 211.122 Materials examination and usage criteria: There shall be written procedures describing in detail the receipt, identification, storage, handling, sampling, examination, and/or testing of labeling and packaging materials ,materials that do not meet such specifications shall be rejected to prevent their use 211.125 Labeling issuance: Labeling materials issued for a batch shall be carefully examined for identity and conformity to the labeling specified in the master or batch production records. 211.130 Packaging and labeling operations: (a) Prevention of mix-ups and cross-contamination by physical separation from operations on other drug products. (b) Identification of the drug product with a lot or control number that permits determination of the history of the manufacture and control of the batch. 211.132 Tamper-resistant packaging requirements for over-the-counter (OTC) human drug products: 211.134 Drug product inspection: 211.137 Expiration dating: (a) To assure that a drug product meets applicable standards of identity, strength, quality, and purity at the time of use. (b) It shall be related to any storage conditions stated on the labeling, as determined by stability studies Subpart H-Holding and Distribution 211.142 Warehousing procedures: (a) Quarantine of drug products before release by the quality control unit. (b) Storage of drug products under appropriate conditions of temperature, humidity, and light so that the identity, strength, quality, and purity of the drug products are not affected.
- 47. 21 (c)Written procedures are necessary. 211.150 Distribution procedures: (a) A procedure whereby the oldest approved stock of a drug product is distributed first. Subpart I-Laboratory Controls 211.160 General requirements: (a) They shall include the establishment of, standards, sampling plans, and test procedures etc to confirm appropriate standards of identity, strength, quality, and purity. (b)The calibration of instruments, apparatus, and recording devices at suitable intervals in accordance to written program 211.165 Testing and release for distribution: (a) The sterility and/or Pyrogen testing are conducted on specific batches of short lived radiopharmaceuticals, 211.166 Stability testing: (a) Stability testing shall be used in determining appropriate storage conditions and expiration dates. (b)Accelerated studies, combined with basic stability information on the components, drug products. 211.167 Special testing requirements: For each batch purporting to be sterile and/or Pyrogen -free, there shall be appropriate laboratory testing to determine conformance to such requirements. 211.173 Laboratory animals: Animals used in testing components, in-process materials, or drug products for compliance with established specifications shall be maintained .They shall be identified, and adequate records shall be maintained showing the history of their use. 211.176 Penicillin contamination:
- 48. 22 (a)If a non-penicillin drug product has been exposed to cross-contamination with penicillin, the non-penicillin drug product shall be tested for the presence of penicillin. (b) Such drug product shall not be marketed if detectable levels are found when tested according to procedures. Subpart J-Records and Reports 211.182 Equipment cleaning and use log: A written record of major equipment cleaning, maintenance and use shall be included in individual equipment logs that show the date, time, product, and lot number of each batch processed. 211.184 Component, drug product container, closure, and labeling records: (a) The identity and quantity of each shipment of each lot of components, drug product containers, closures, and labeling; the name of the supplier. 211.186 Master production and control records: To assure uniformity from batch to batch and including each batch size thereof, shall be prepared, dated, and signed by one person and independently checked, dated, and signed by a second person. 211.188 Batch production and control records: Batch production and control records shall be prepared for each batch produced and shall include complete information relating to the production and control of each batch. (a) An accurate reproduction of the appropriate master production or control record, checked for accuracy, dated, and signed; (b) Documentation that each significant step in the manufacture, processing, packing, or holding of the batch was accomplished 211.192 Production record review: All drug product, production and control records, including those for packaging and labeling, shall be reviewed and approved by the quality control unit to determine compliance with all established, approved written procedures before a batch is released or distributed.
- 49. 23 211.194 Laboratory records: It shall include complete data derived from all tests 211.196 Distribution records: It shall contain the name and strength of the product and description of the dosage form, name and address of the consignee, date and quantity shipped, and lot or control number of the drug product. 211.198 Complaint files: All written and oral complaints regarding a drug product shall be established and followed. Such procedures shall include provisions for review by the quality control unit, of any complaint involving the possible failure of a drug product to meet any of its specifications Subpart K-Returned and Salvaged Drug Products 211.204 Returned drug product: (a) Products shall be identified as such and held. If the conditions under which returned drug products have been held, stored, or shipped before or during their return (b) if the condition of the drug product, its container, carton, or labeling , as a result of storage or shipping, casts doubt on the safety, identity, strength, quality or purity of the drug product 211.208 Drug product salvaging: Drug products that have been subjected to improper storage conditions including extremes in temperature, humidity, equipment failures shall not be salvaged and returned to the marketplace.
- 50. 24 CENTRE FOR DRUG EVALUATION AND RESEARCH (CDER) CDER : The Center for Drug Evaluation and Research (CDER) is a division of the U.S. Food and Drug Administration (FDA) that monitors most drugs as defined in the Food, Drug, and Cosmetic Act. CDER Mission : ➢ CDER’s mission is to protect and promote public health by helping to ensure that human drugs are safe and effective for their intended use. ➢ To protect public health by promoting the safe use of marketed drugs and by helping to ensure the quality and integrity of marketed drug products. DEPARTMENT OF HEALTH AND HUMAN SERIVICES FOOD AND DRUG ADMINISTRATION (FDA) Center for Drug Evaluation & Research (CDER) Center for Biologics Evaluation & Research (CBER) Center for Devices & Radiological Health (CDRH) Center for Food Safety & Applied Nutrition (CFSAN) Center for Veterinary Medicine (CVM) National Center for Toxicological Research Office of Regulatory Affairs Office of the Commissioner