Orbital diagram principles and hybridization in chemistry
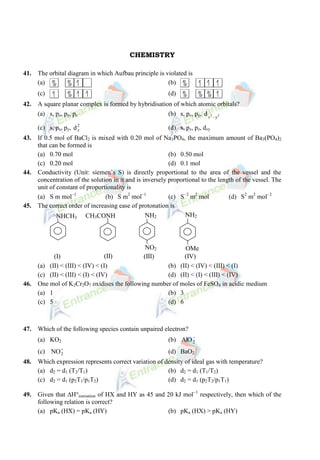
- 1. CHEMISTRY 41. The orbital diagram in which Aufbau principle is violated is (a) (b) (c) (d) 42. A square planar complex is formed by hybridisation of which atomic orbitals? (a) s, px, py, pz (b) s, px, py, 2 2 y d x (c) s, px, py, 2 z d (d) s, px, pz, dxy 43. If 0.5 mol of BaCl2 is mixed with 0.20 mol of Na3PO4, the maximum amount of Ba3(PO4)2 that can be formed is (a) 0.70 mol (b) 0.50 mol (c) 0.20 mol (d) 0.1 mol 44. Conductivity (Unit: siemen’s S) is directly proportional to the area of the vessel and the concentration of the solution in it and is inversely proportional to the length of the vessel. The unit of constant of proportionality is (a) S m mol 1 (b) S m2 mol 1 (c) S 2 m2 mol (d) S2 m2 mol 2 45. The correct order of increasing ease of protonation is NHCH3 (I) (II) (III) (IV) NO2 OMe CH3CONH NH2 NH2 (a) (II) < (III) < (IV) < (I) (b) (II) < (IV) < (III) < (I) (c) (II) < (III) < (I) < (IV) (d) (II) < (I) < (III) < (IV) 46. One mol of K2Cr2O7 oxidises the following number of moles of FeSO4 in acidic medium (a) 1 (b) 3 (c) 5 (d) 6 47. Which of the following species contain unpaired electron? (a) KO2 (b) 2 AlO (c) 2 NO (d) BaO2 48. Which expression represents correct variation of density of ideal gas with temperature? (a) d2 = d1 (T2/T1) (b) d2 = d1 (T1/T2) (c) d2 = d1 (p2T1/p1T2) (d) d2 = d1 (p2T2/p1T1) 49. Given that H°ionisation of HX and HY as 45 and 20 kJ mol 1 respectively, then which of the following relation is correct? (a) pKa (HX) = pKa (HY) (b) pKa (HX) > pKa (HY)
- 2. (c) pKa (HX) < pKa (HY) (d) pKa (HX) = pKa (HY) + 20 45 log 50. One mole of N2H4 loses 10 mole of electrons to form a new compound Y. Assuming that all nitrogen appears in the new compound, what is the oxidation number of nitrogen in Y (There is no change in the oxidation state of hydrogen)? (a) 3 (b) +3 (c) +5 (d) +1 51. 0.5 mole of each of H2, SO2 and CH4 are kept in a container. A hole was made in the container. After 3 hours, the order of partial pressures in the container will be (a) SO2 P > CH4 P > H2 P (b) H2 P > SO2 P > CH4 P (c) CH4 P > SO2 P > H2 P (d) CH4 P > H2 P > SO2 P 52. On bromination propionic acid yields two isomeric 2 bromopropionic acids. This pair will be an example of (a) optical isomers. (b) cis trans isomers. (c) chain isomers. (d) position isomers. 53. Which of the following set represents increasing dipole moment of? (I) Chlorobenzene (II) m dichlorobenzene (III) o dichlorobenzene (IV) p dichlorobenzene (a) (I) < (IV) < (II) < (III) (b) (IV) < (I) < (II) < (III) (c) (IV) < (I) < (III) < (II) (d) (IV) < (II) < (I) < (III) 54. Which is the correct order of acidic strength of the following acids? (a) CH3CH2COOH > CH2=CH COOH > CH C COOH (b) CH3CH2COOH < CH2=CH COOH < CH C COOH (c) CH3CH2COOH < CH2=CH COOH > CH C COOH (d) CH3CH2COOH > CH2=CHCOOH < CH C COOH 55. The standard electrode potential (E°) for OCl /Cl and Cl / 2 1 Cl2 respectively are 0.94 V and 1.36 V. The E° value of OCl / 2 1 Cl2 will be (a) 0.42 V (b) 2.20 V (c) 0.52 V (d) 1.04 V 56. For a reaction, SO2(g) + O (g) 2 1 2 SO3(g) if Kp = 1.7 × 1012 at 20°C and 1 atm pressure then value of Kc is (a) 1.7 × 1012 (b) 0.7 × 1012 (c) 8.31 × 1012 (d) 1.2 × 1012 57. In the spectrochemical series which ligand produces strong field?
- 3. (a) Cl (b) H2O (c) NO2 (d) CO 58. Which of the following is used for dry bleaching? (a) Chlorine (b) SO2 (c) Ozone (d) H2O2 59. Out of the following, the metals that cannot be obtained by the electrolysis of the aqueous solution of their salts are (a) Ag (b) Mg (c) Cu (d) Al 60. Plot of log (a x) vs time t is a straight line. This indicates that the reaction is of (a) second order (b) first order (c) zero order (d) third order 61. The enthalpy of formation of SO2(g) is 296.7 kJ. The energy required for the decomposition of 2 mole of SO2(g) into sulphur(rhombic) and gaseous O2 is (a) 148.35 kJ (b) 296.7 kJ (c) 593.4 kJ (d) 148.35 kJ 62. At room temperature HCl is a gas while HF is a low boiling liquid. This is because (a) H F bond is covalent (b) H F bond is ionic. (c) H F has metallic bond. (d) H F has hydrogen bond. 63. The helical structure of proteins is stabilized by (a) Peptide bonds (b) Dipeptide bond (c) Hydrogen bond (d) vander Waals forces 64. The ratio between the r.m.s velocity of H2 at 50 K and that of O2 at 800 K is (a) 4 (b) 2 (c) 1 (d) 1/4 65. An organic compound ‘X’ is boiled with KOH when it evolves a gas, which gives white precipitate with MgSO4 solution. The compound ‘X’ also gives salicyclic acid on warming with phenol. ‘X’ is likely to be (a) C6H5Cl (b) CCl4 (c) CHCl3 (d) C6H5OH 66. In which of the following Cl is not easily replaced? (a) CH3CH2Cl (b) Picryl chloride (c) Vinyl chloride (d) Allyl chloride 67. 2 moles of acetaldehyde are warmed in the presence of 10% Ba(OH)2 to give a product which dehydrates to a compound ‘X’. The compound on reduction with NaBH4 will give (a) CH3CH=CHCHO (b) CH3CH=CHCH2OH (c) CH3CH2CH2CH2OH (d) CH3CHCH2CH2OH OH 68. Benzene ICl AlCl3 A ‘A’ is:
- 4. (a) Cl (b) I (c) I Cl (d) I C l 69. Length of a unit cell edge of the bcc lattice metal is 200 nm. Then the radius of the atom of the metal is (a) 89.1 nm (b) 86.6 nm (c) 78.3 nm (d) 98.3 nm 70. Which of the following monomers can undergo free radical, cationic as well as anionic polymerisation with equal ease? (a) CH3–C=CH2 CH3 (b) C6H5–CH=CH2 (c) CH2=CH–CN (d) CH2=CH2 71. The bond energy of an O H bond is 109 kcal mol 1. When a mole of water is formed from H and O atoms, then (a) 218 kcal is released. (b) 109 kcal is released. (c) 218 kcal is absorbed. (d) 109 kcal is absorbed. 72. A solution of Na2SO4 in water is electrolysed using inert electrodes. The product at cathode and anode are respectively (a) H2, O2 (b) O2, H2 (c) O2, Na (d) O2, SO2 73. For the hypothetical reaction, A + B C + D, the rate = k[A]–1/2[B]3/2. On doubling the concentration of A and B, the rate will be (assume that concentration of A and B initially were same) (a) 4 times (b) 2 times (c) 3 times (d) none of these 74. Which of the following substance gives a positively charged sol? (a) Gold (b) Arsenious sulphide (c) Starch (d) Ferric hydroxide 75. The correct order of first ionization potential of the element is: (a) Na > Mg > Al (b) Mg > Na > Al (c) Al > Mg > Na (d) Mg > Al > Na 76. For the reaction, C H OH HX C H X 2 5 ZnX 2 5 2 . The decreasing order of reactivity of halogen acids is : (a) HI > HCl > HBr (b) HI > HBr > HCl (c) HCl > HBr > HI (d) HBr > HI > HCl 77. CH CHO X Oxidation SeO 3 2 . The product X of the reaction is: (a) CO2 + H2O (b) OHC–CHO (c) (COOH)2 (d) (CH3)2CO 78. When phenolic ether is heated with HI, it yields (a) alkyl halide + phenol (b) alcohol + aryl halides
- 5. (c) alkyl halide + aryl halide + H2O (d) aryl halide only 79. Isopropyl amine HO3 KMnO4[X] [Y]. In the above sequence [X] and [Y] are respectively (a) Acetaldimine, Ethanal (b) Ethanal, ketimine (c) Ketimine, acetone (d) Acetone, propan-2-ol 80. Which of the following is a pseudohalogen? (a) IF7 (b) (CN)2 (c) ICl2 (d) 3 I