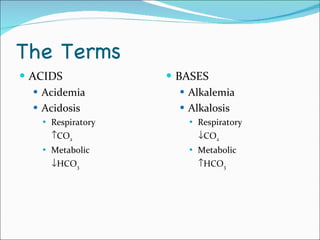
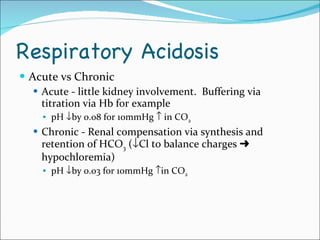
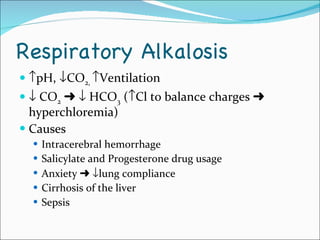
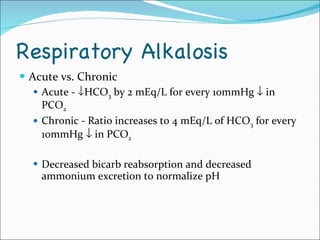
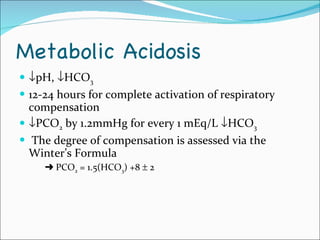
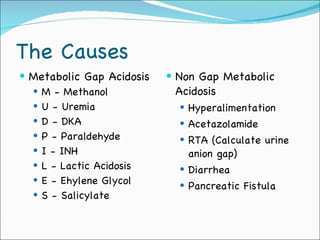
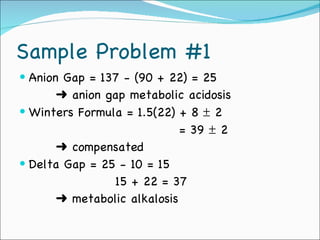
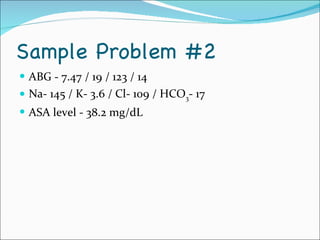
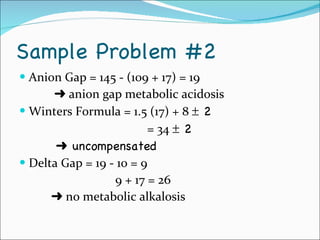
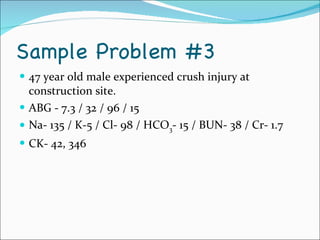
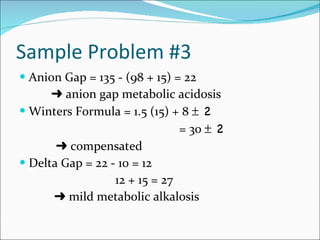
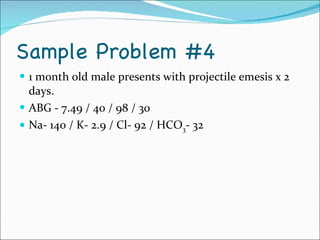
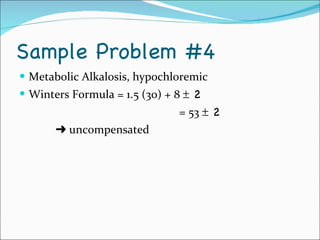
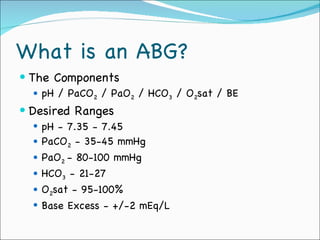
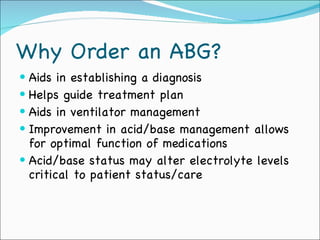
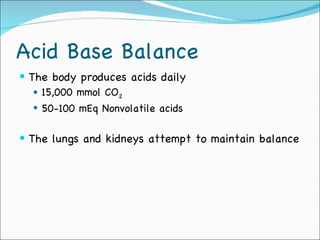
This document provides information about arterial blood gases (ABGs), including the components measured in an ABG, reasons for ordering an ABG, acid-base balance and disorders, and examples of how to analyze ABG results. It defines the components of an ABG as pH, PaCO2, PaO2, HCO3, O2 sat, and BE. Acid-base disorders are classified as respiratory (affecting PaCO2) or metabolic (affecting HCO3). Mixed disorders can involve both. Case examples demonstrate analyzing ABG results to identify acid-base disorders and degree of compensation.


![Acid Base Balance Assessment of status via bicarbonate-carbon dioxide buffer system CO 2 + H 2 O <--> H 2 CO 3 <--> HCO 3 - + H + ph = 6.10 + log ([HCO 3 ] / [0.03 x PCO 2 ])](https://image.slidesharecdn.com/abgpresentation-111031083223-phpapp01/85/Abg-presentation-6-320.jpg)