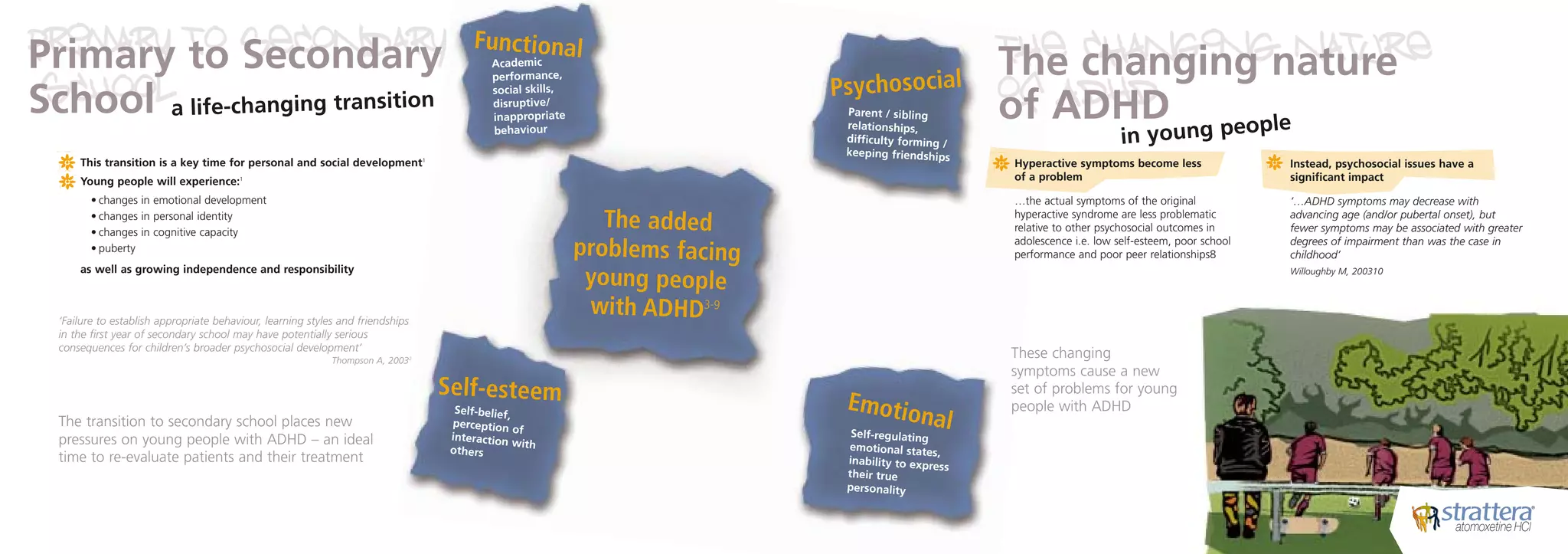
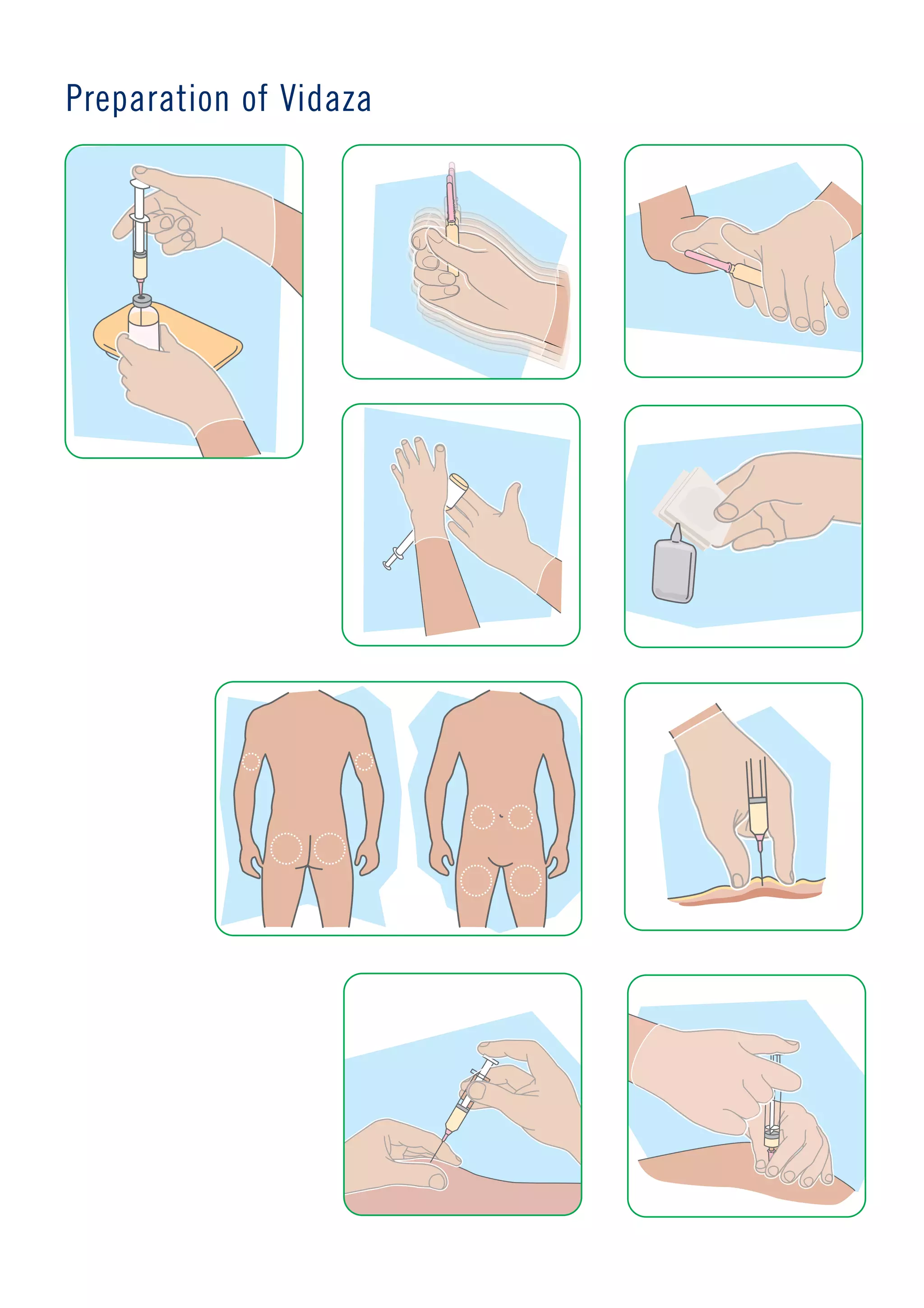
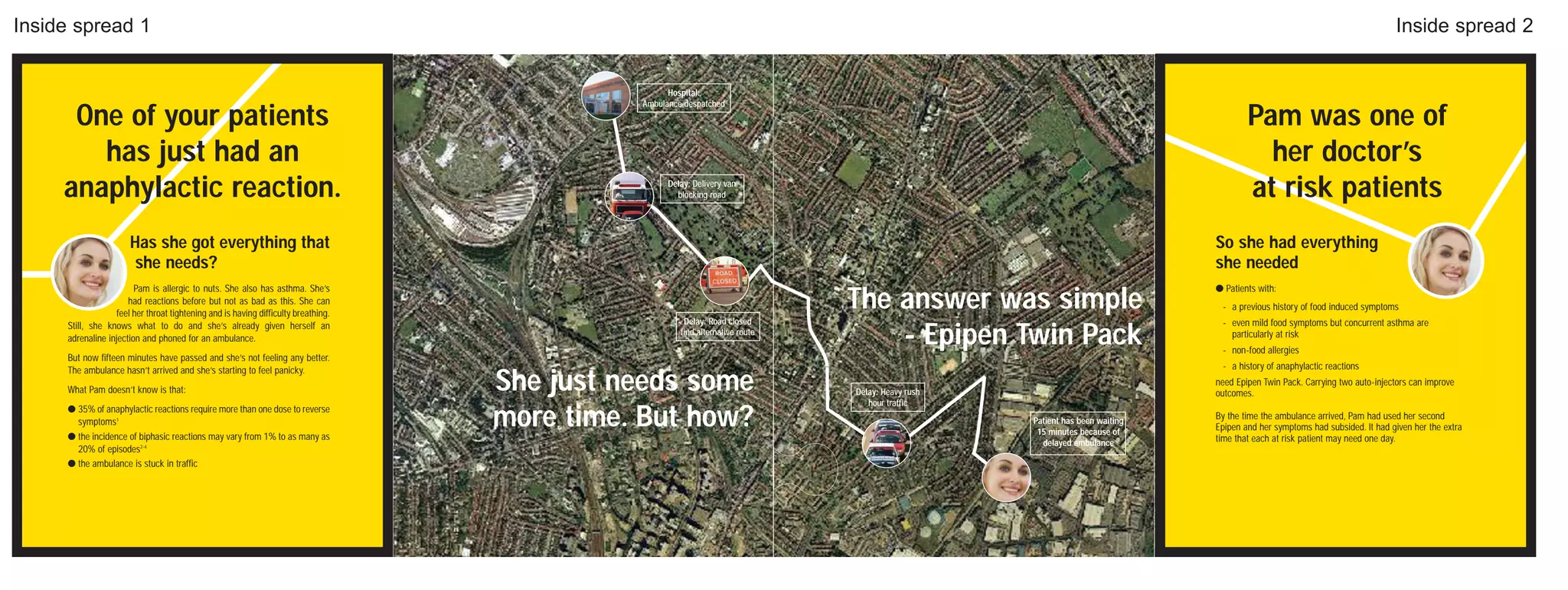
The document discusses weight loss surgery as a solution for people who have tried everything else to lose weight but have had long-term failure. It describes the local BMI hospital as providing personal care and support from specialists before, during, and after treatment to help patients stay healthy and achieve the best results. It encourages readers to contact the hospital soon to change their life for the better.