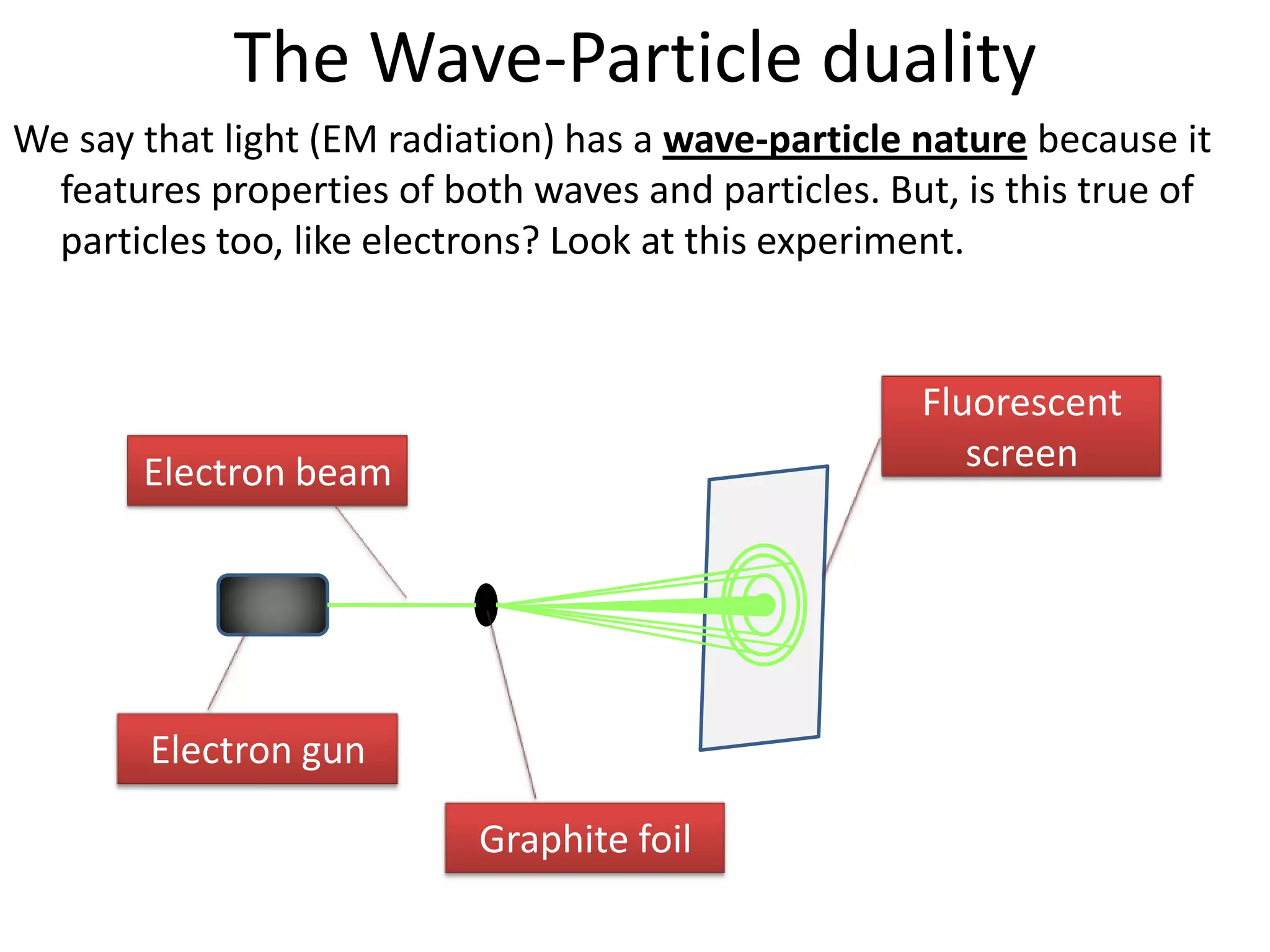
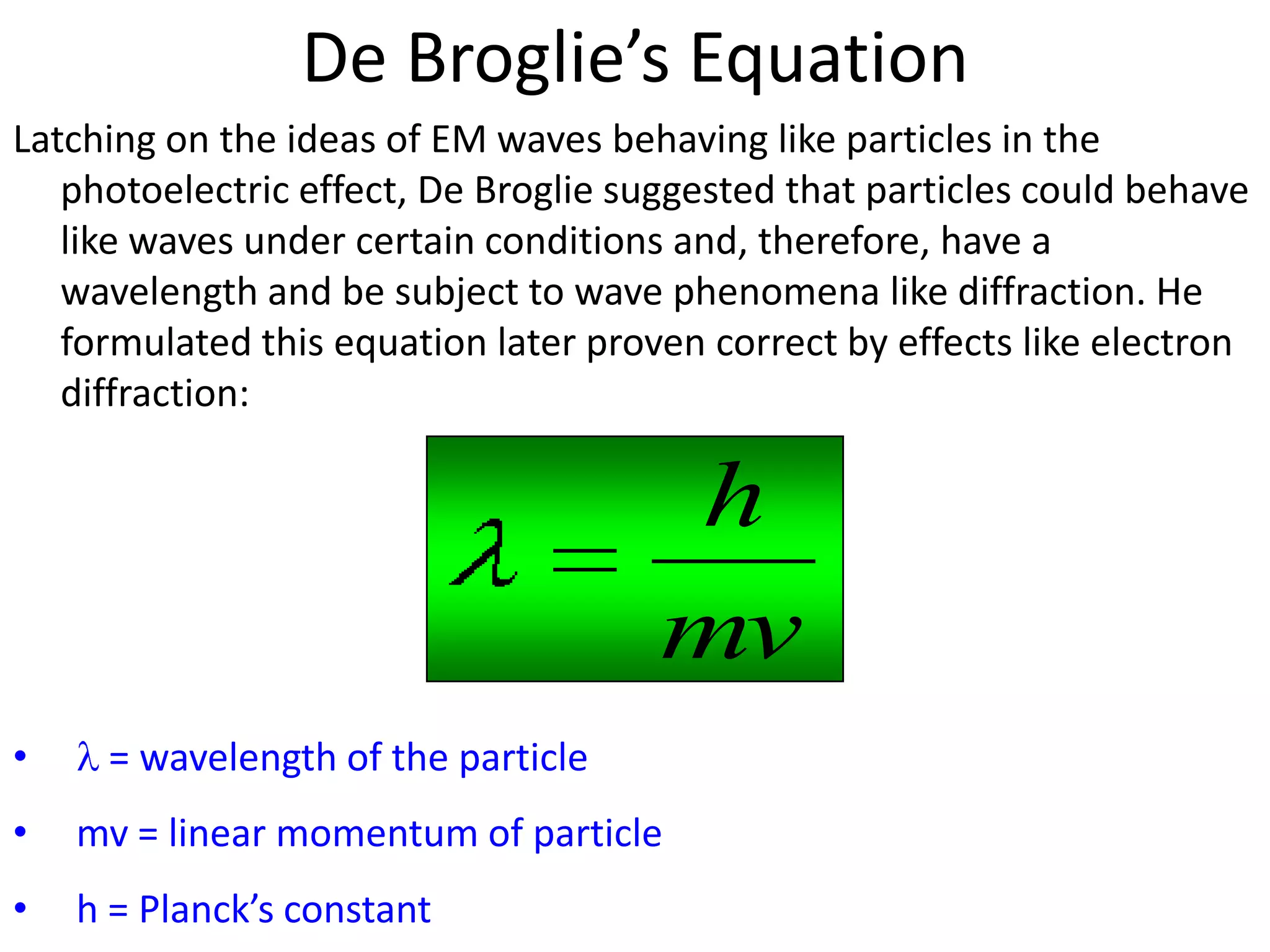
This document discusses the wave-particle duality of light and matter. It explains how experiments demonstrating the photoelectric effect and electron diffraction show that electromagnetic radiation and electrons exhibit both wave-like and particle-like properties depending on the situation. De Broglie hypothesized that all particles can behave as waves, and he formulated an equation showing that particles are associated with a wavelength determined by their momentum and Planck's constant.