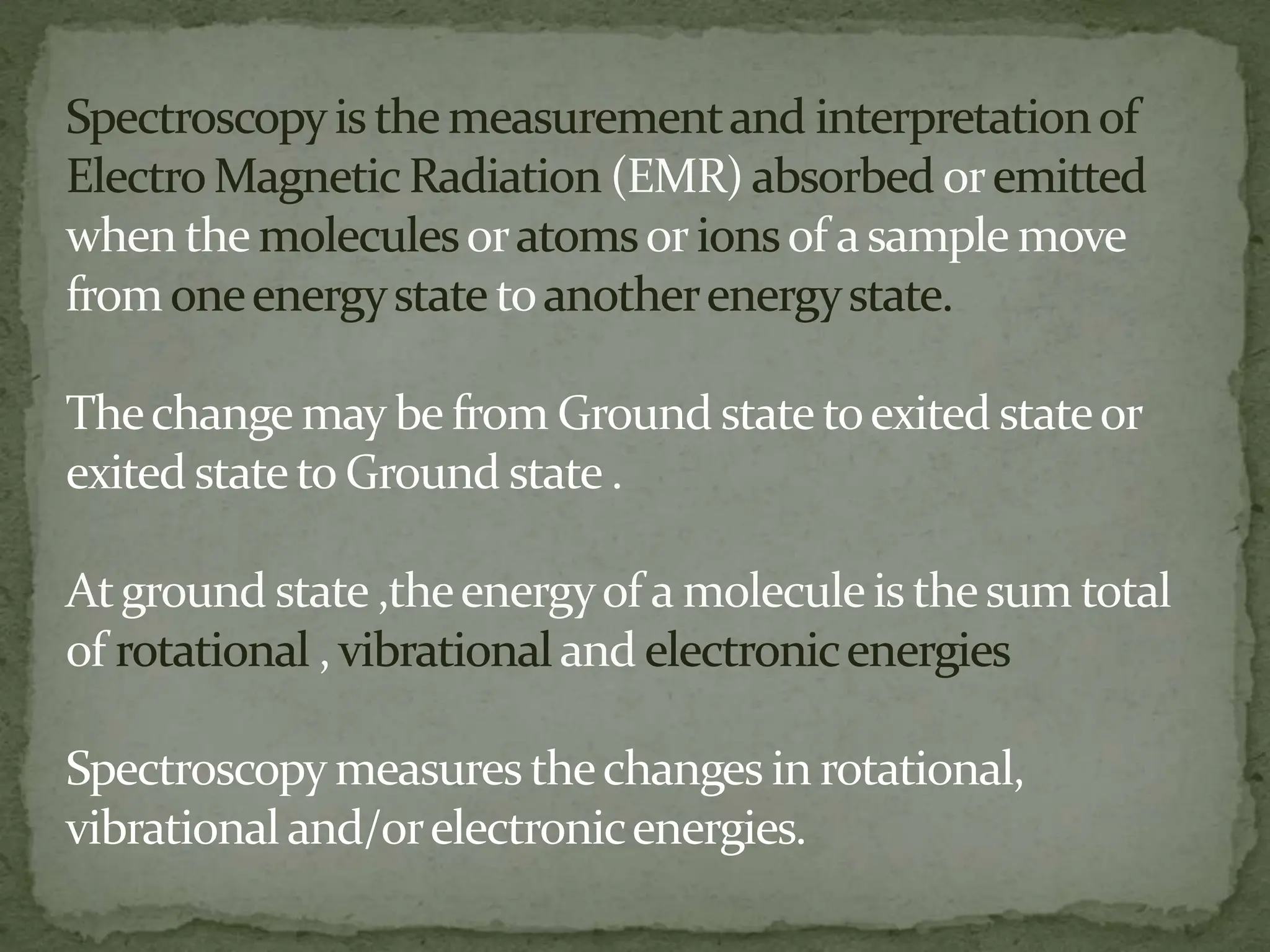
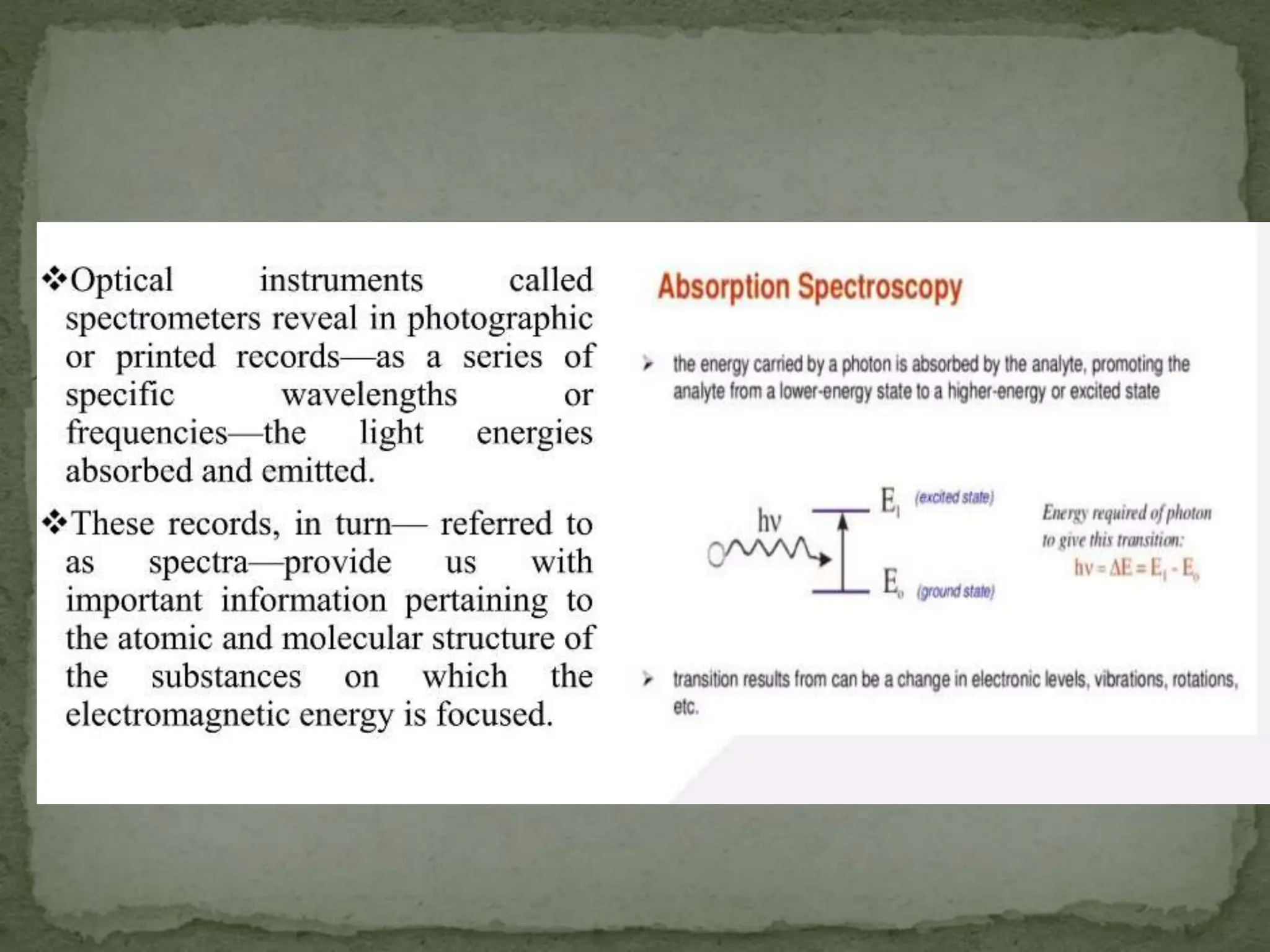
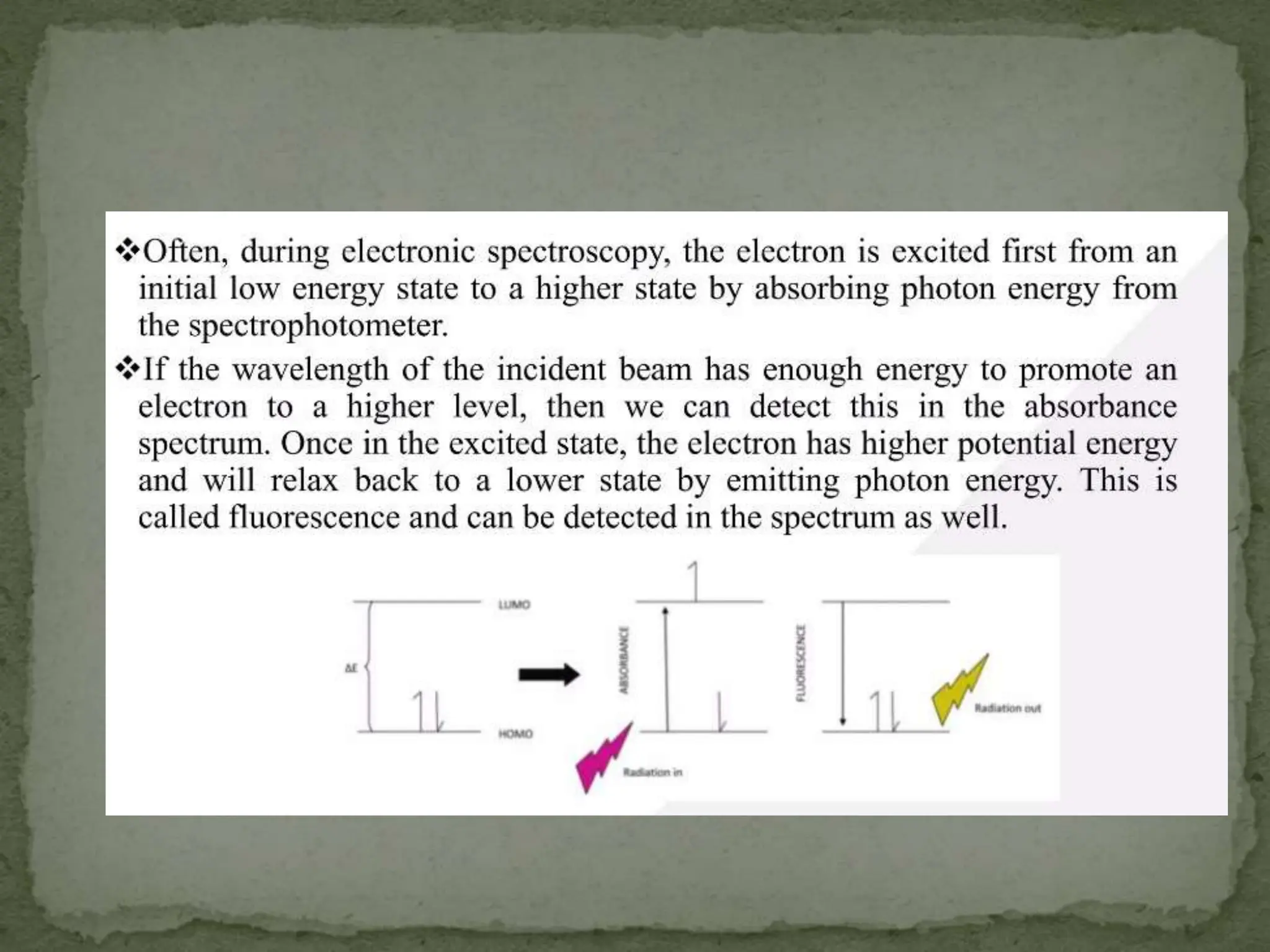
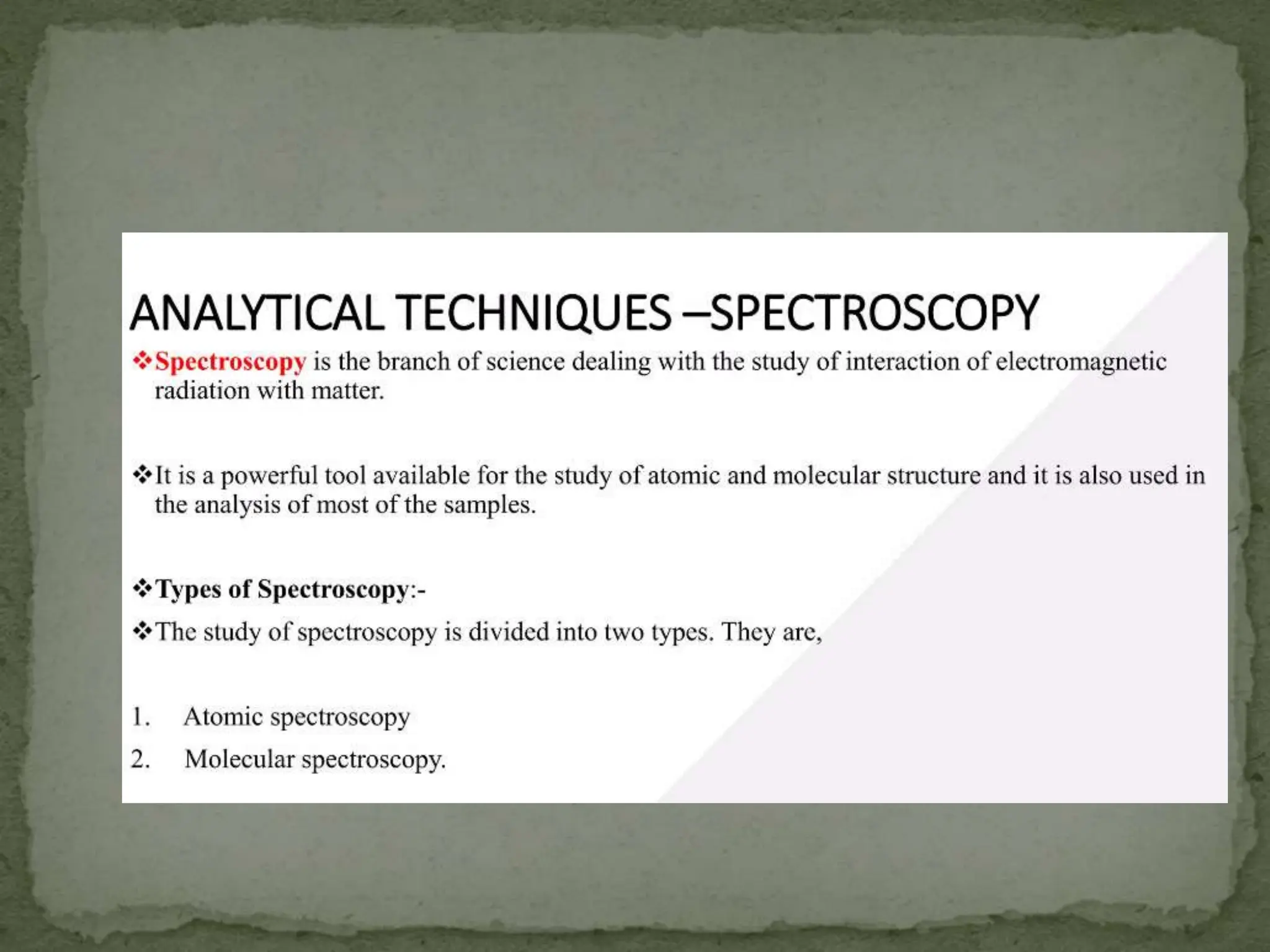
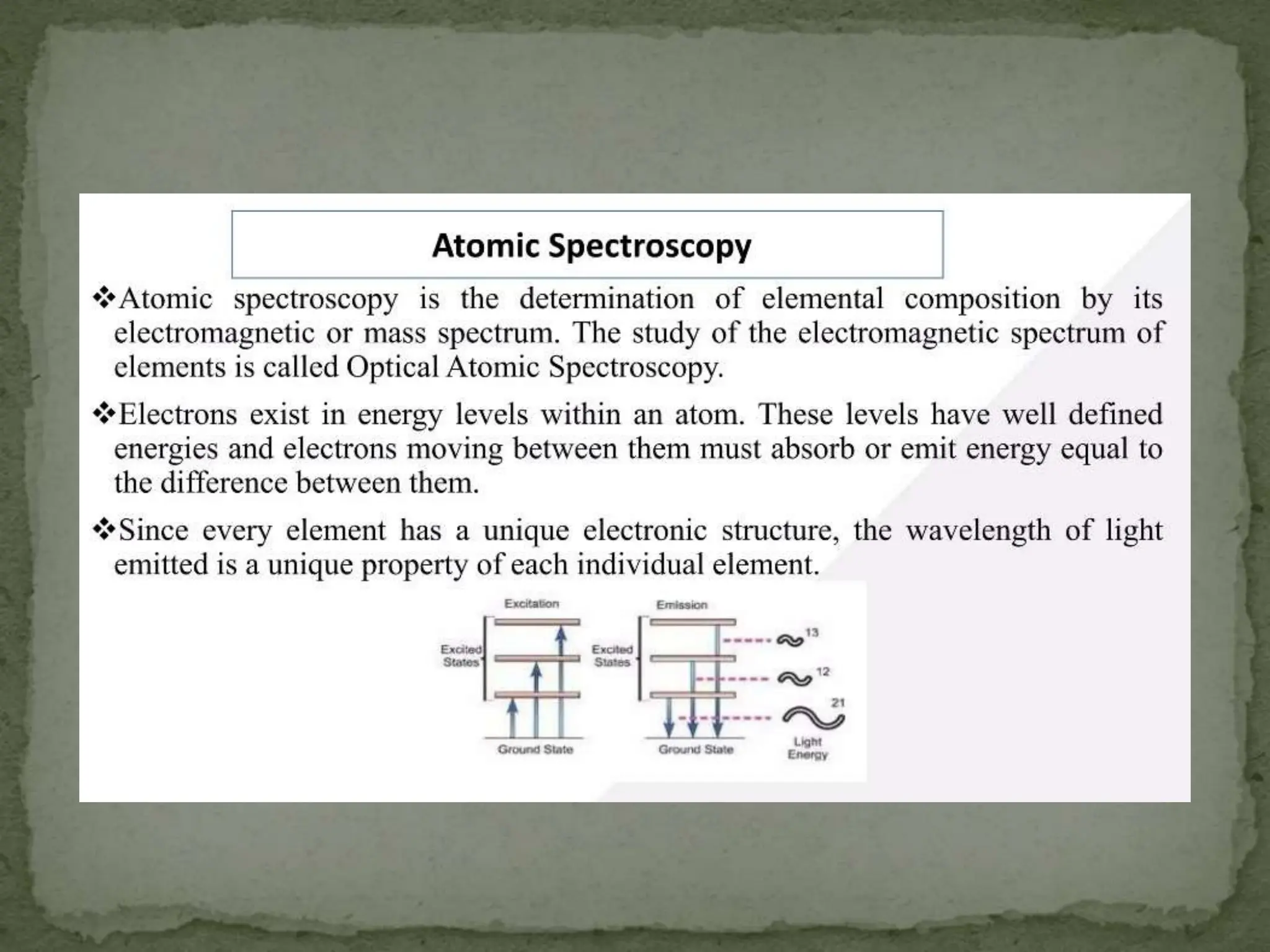
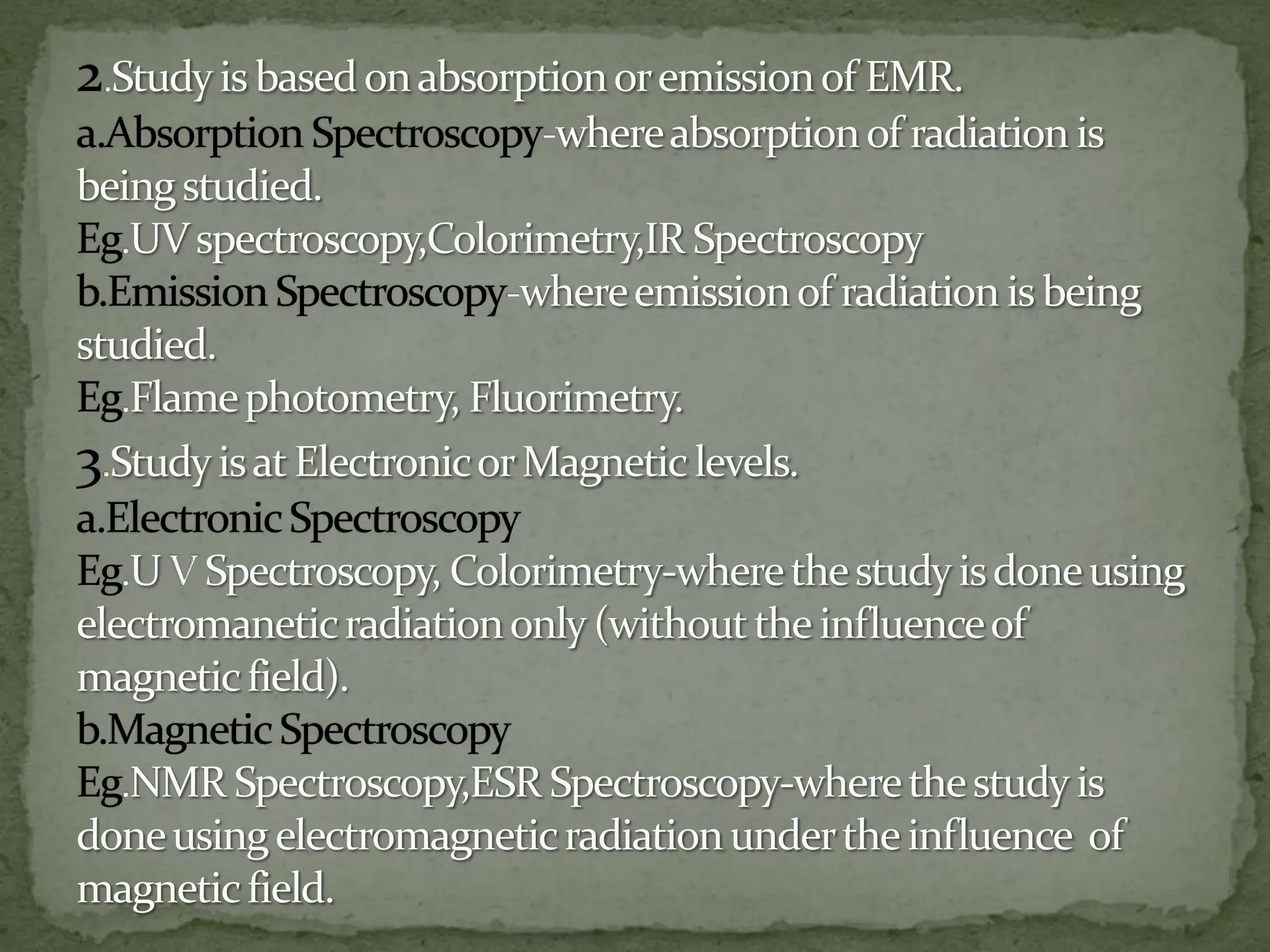
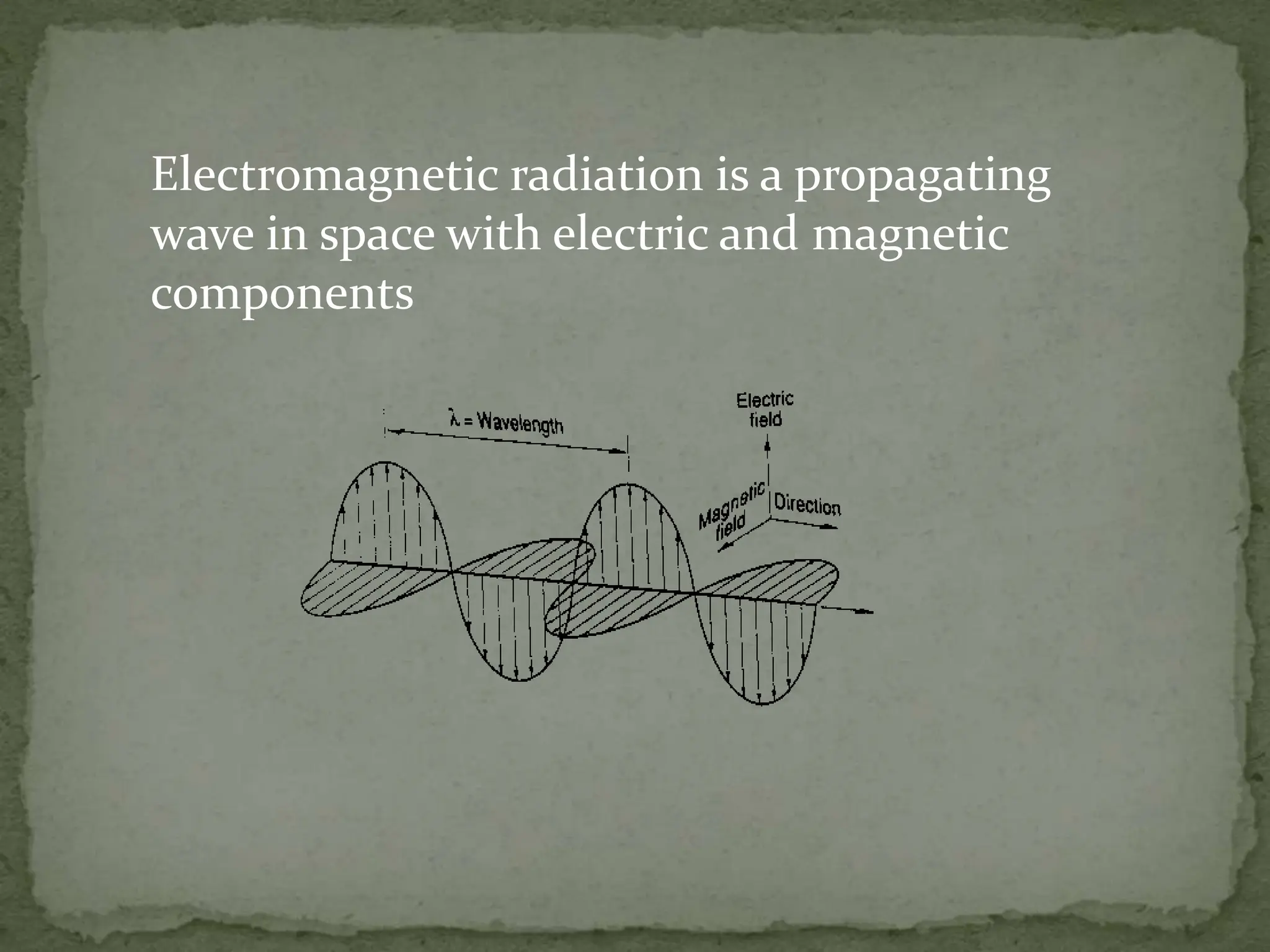
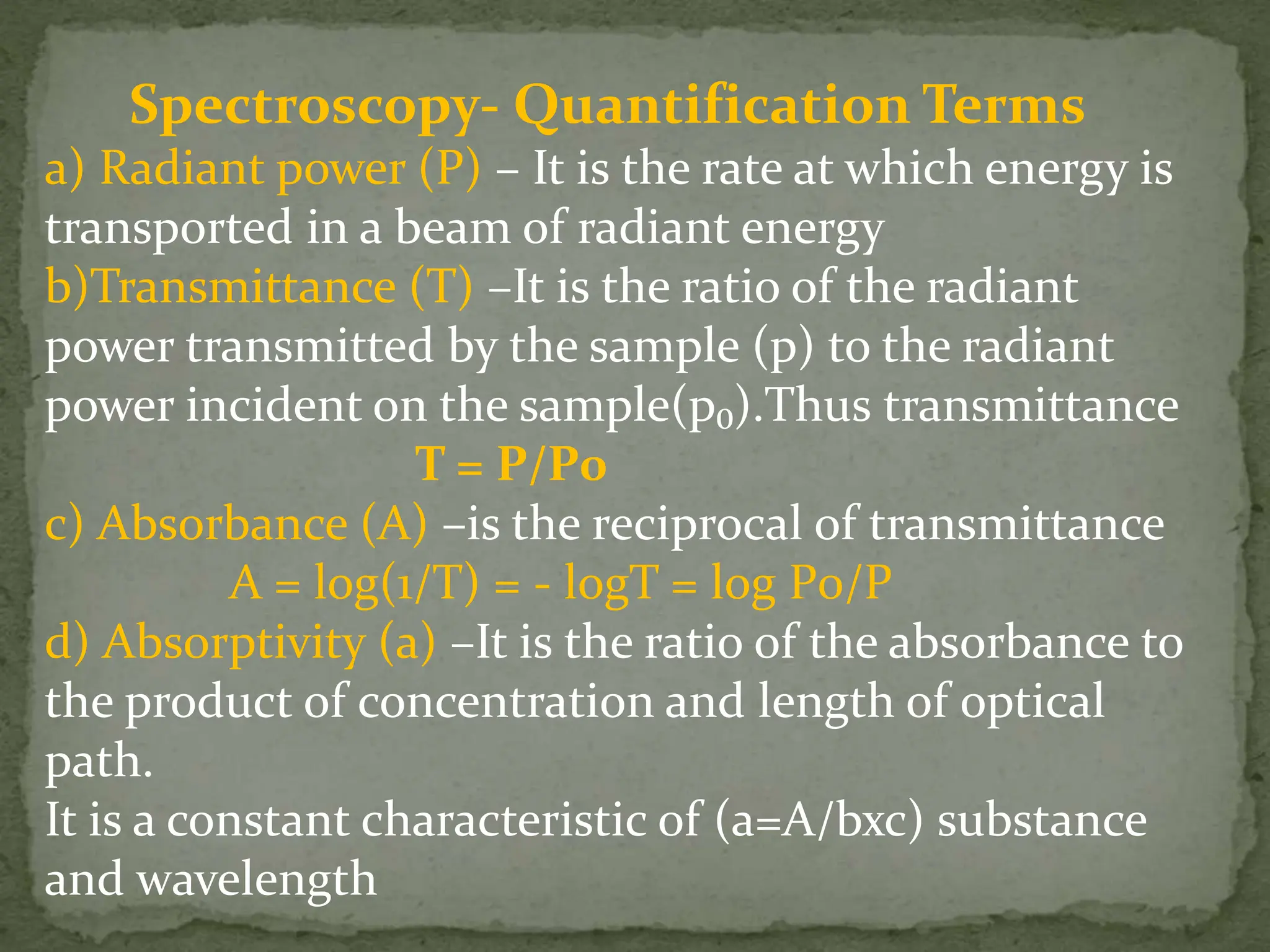
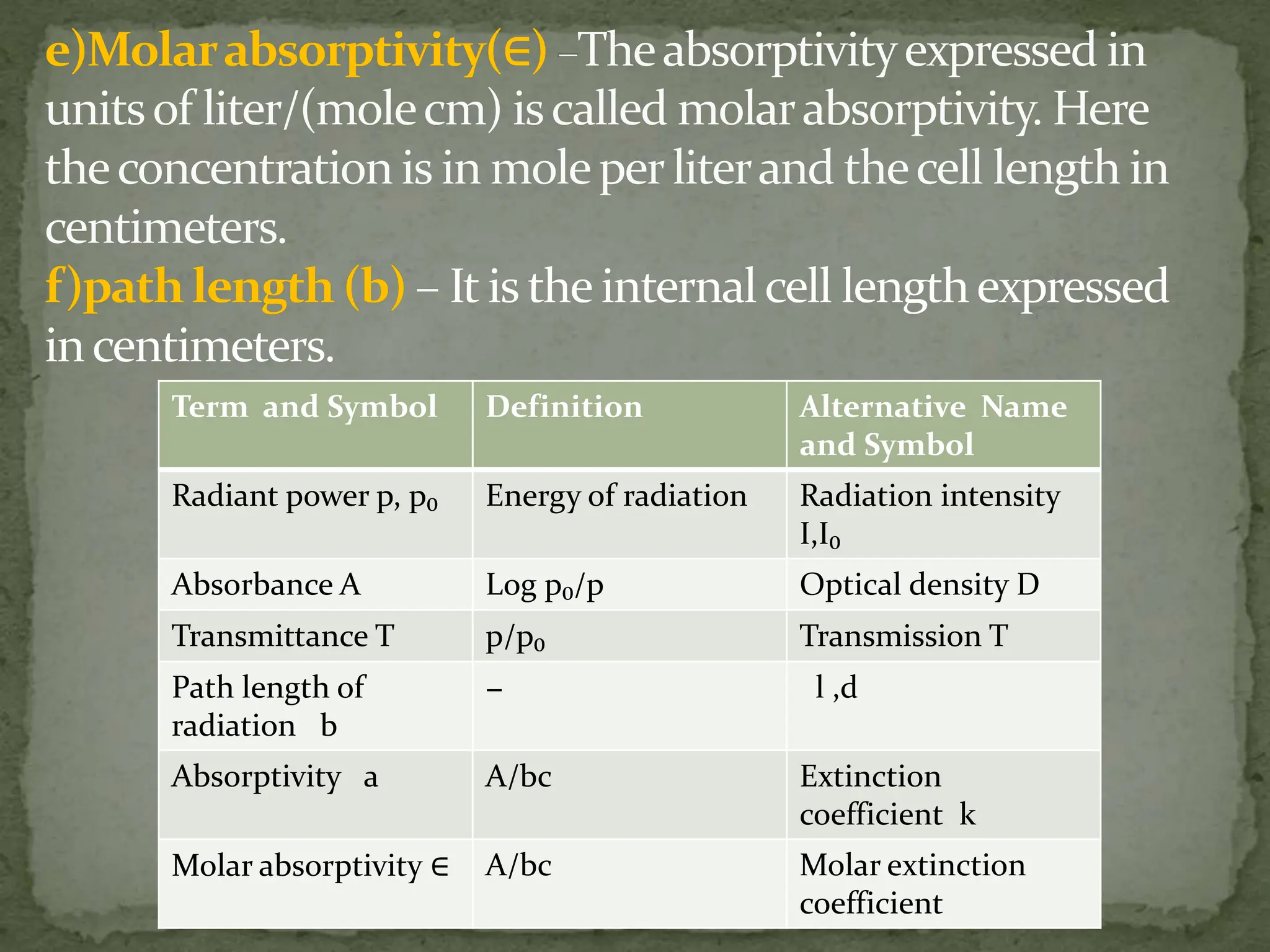
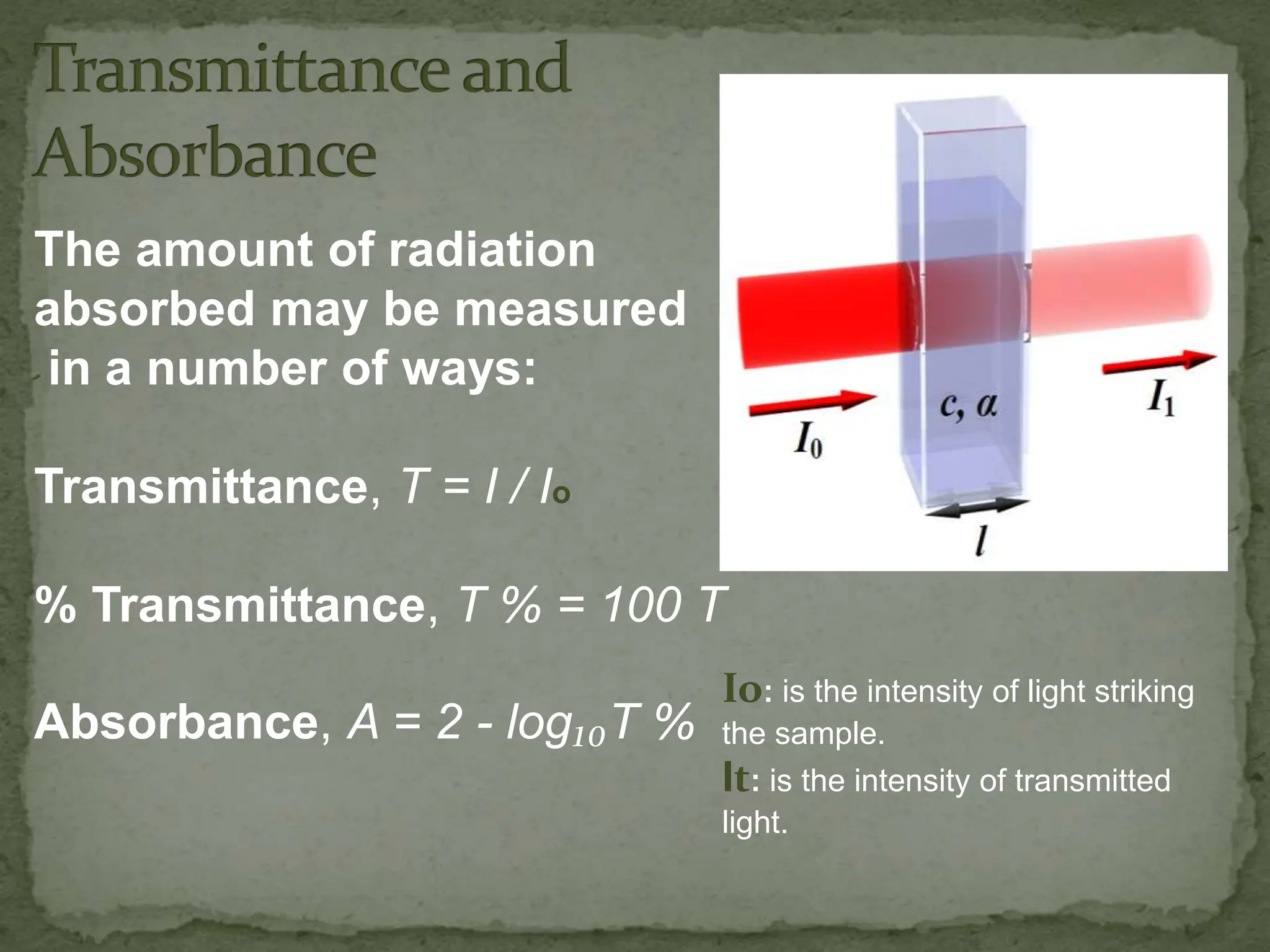
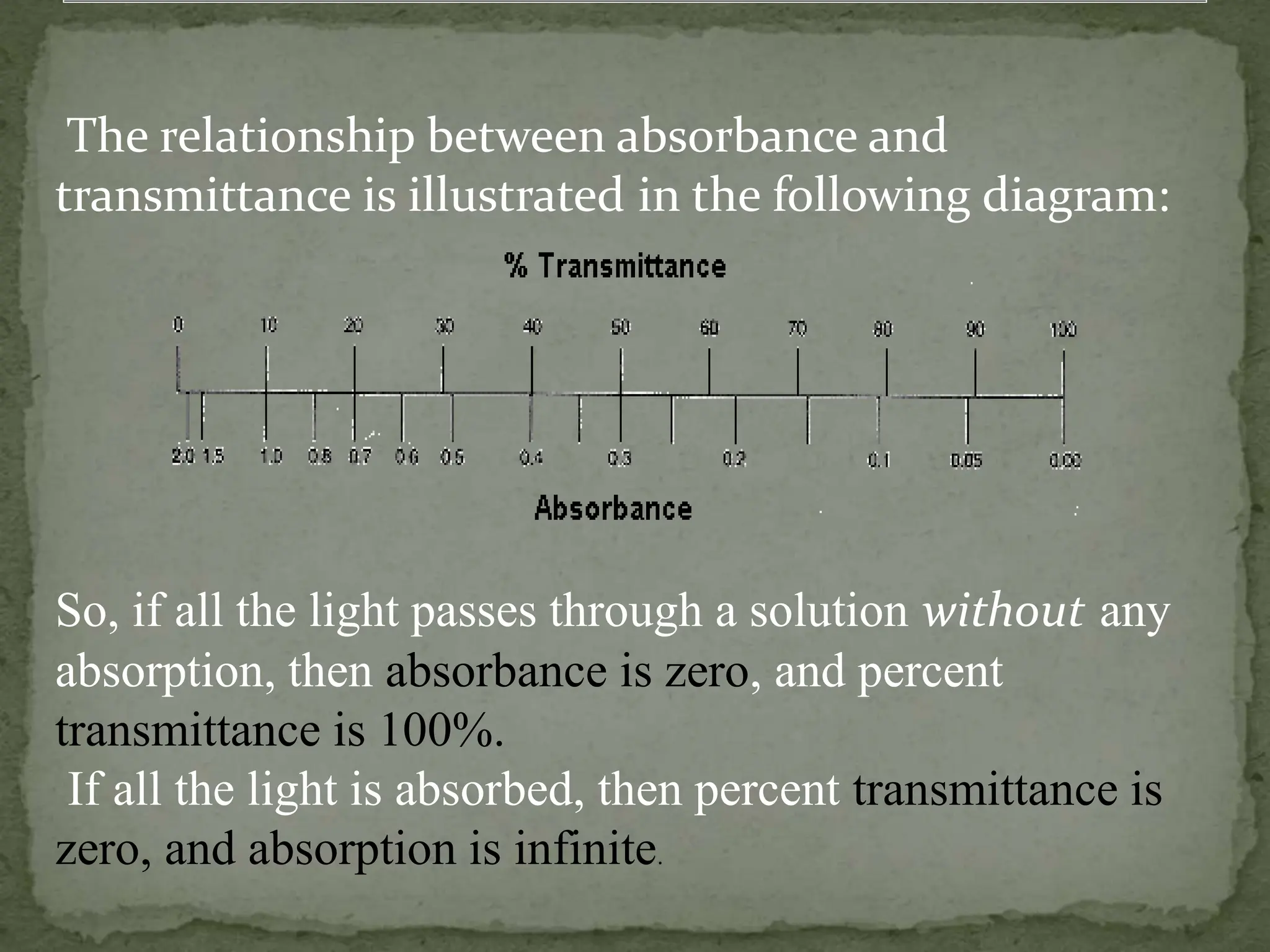
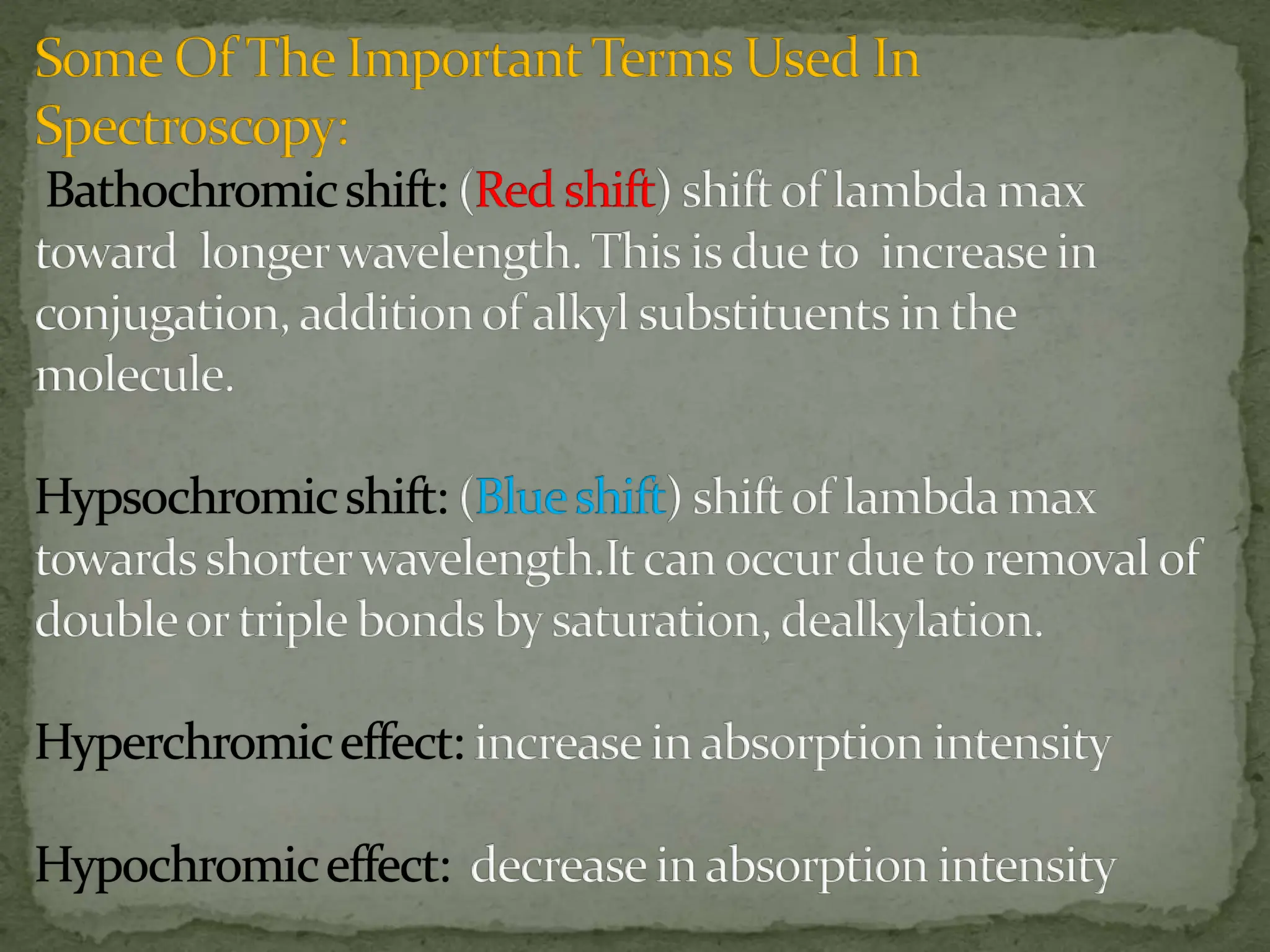
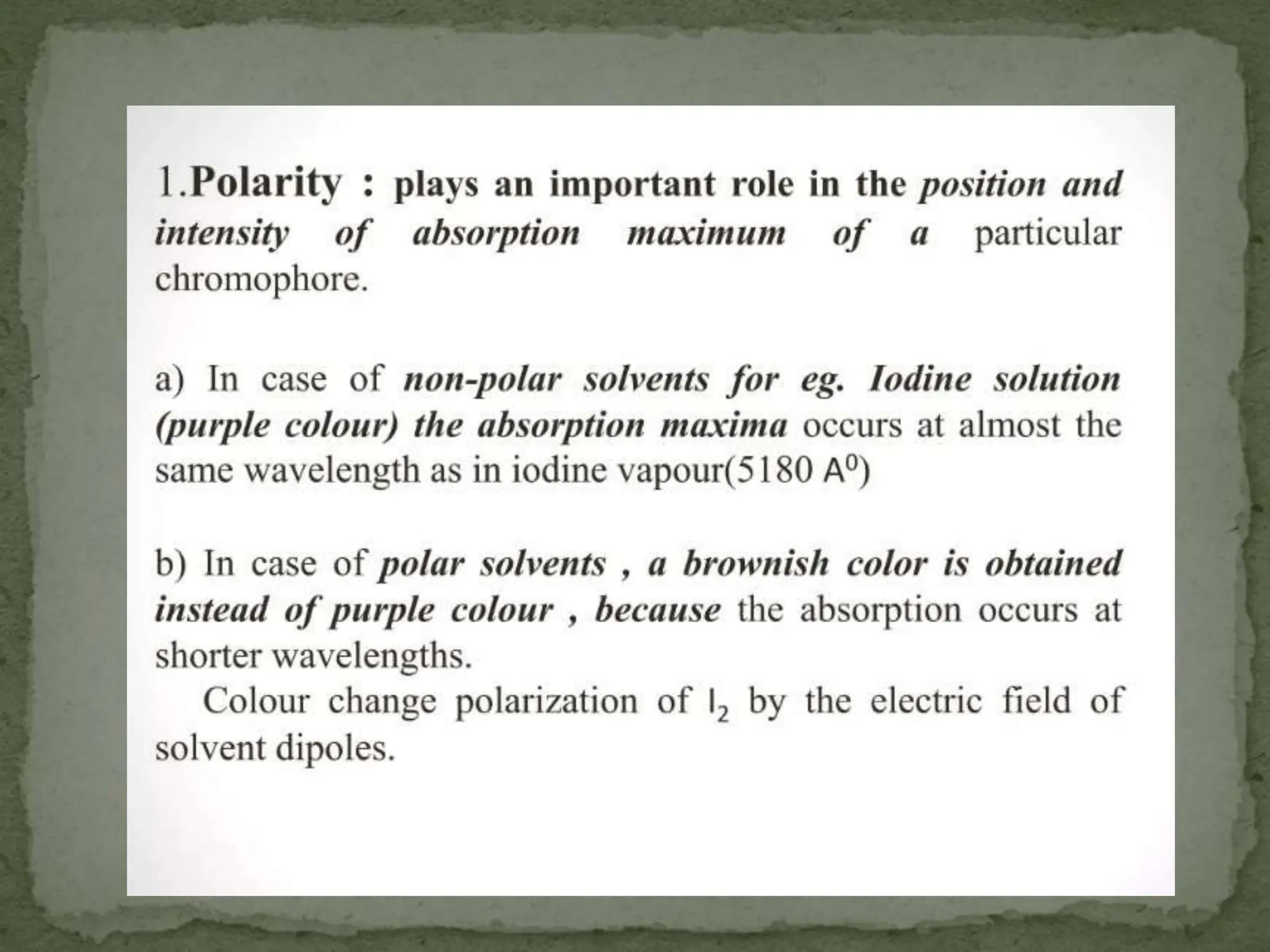
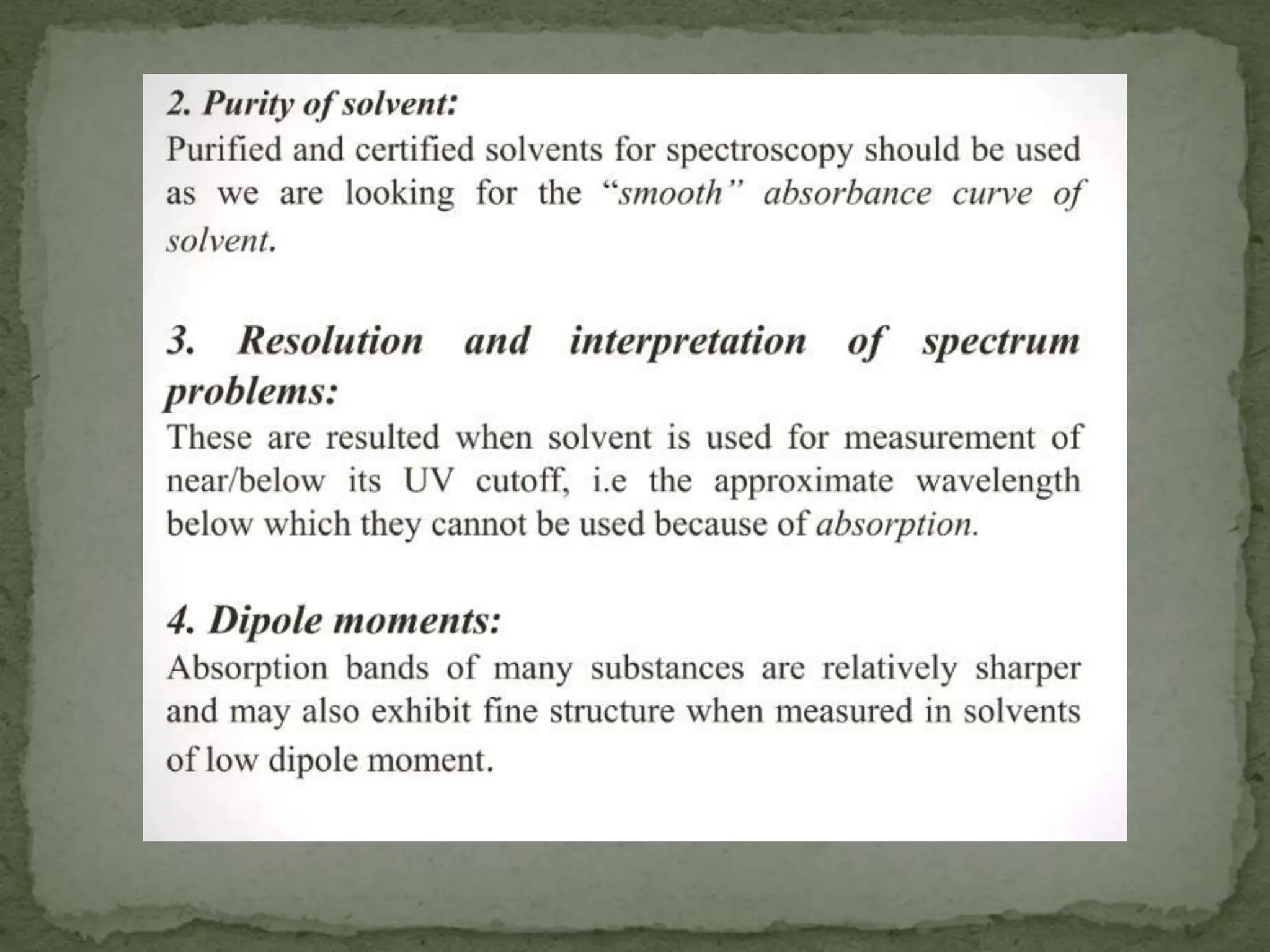
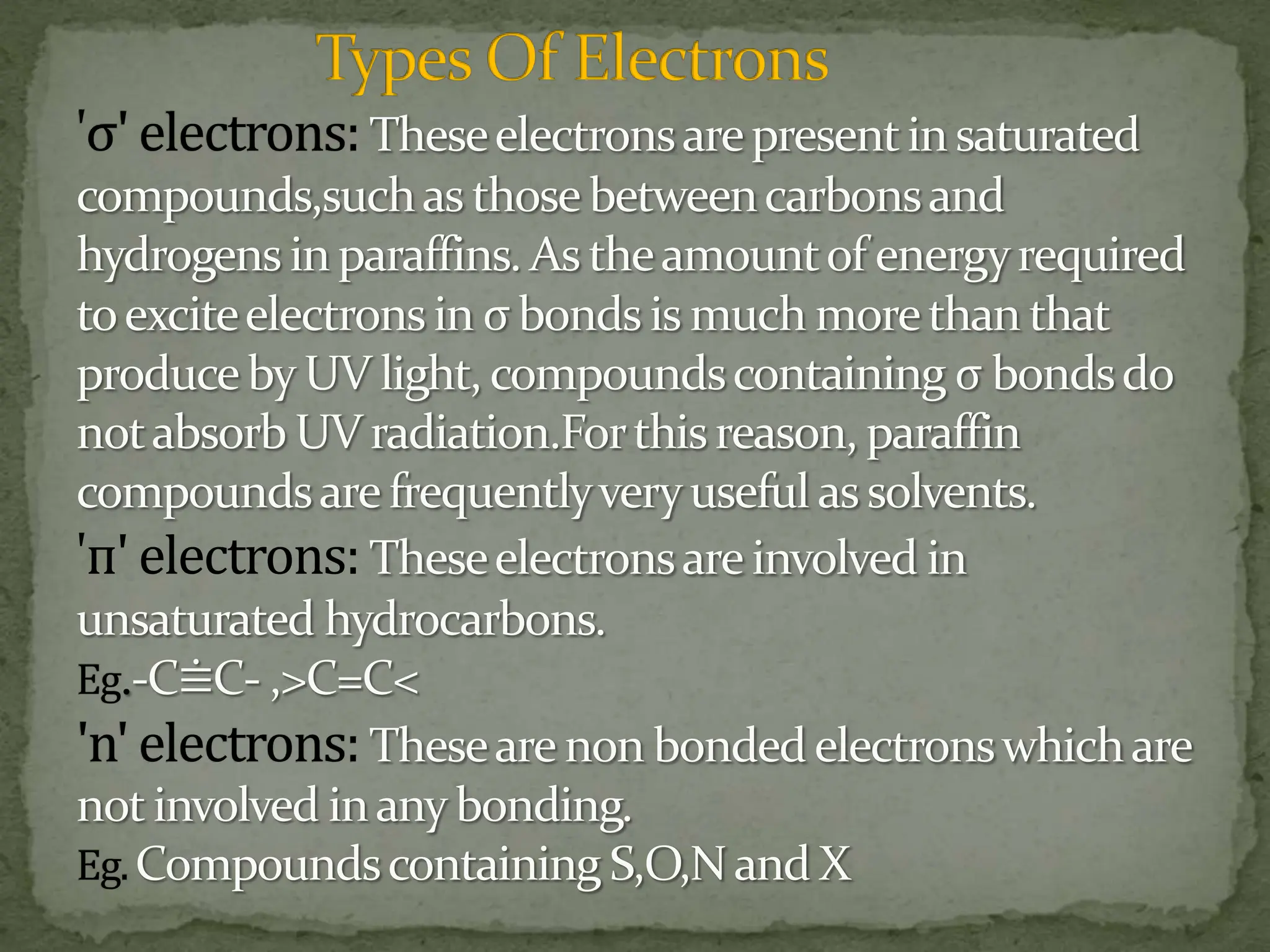
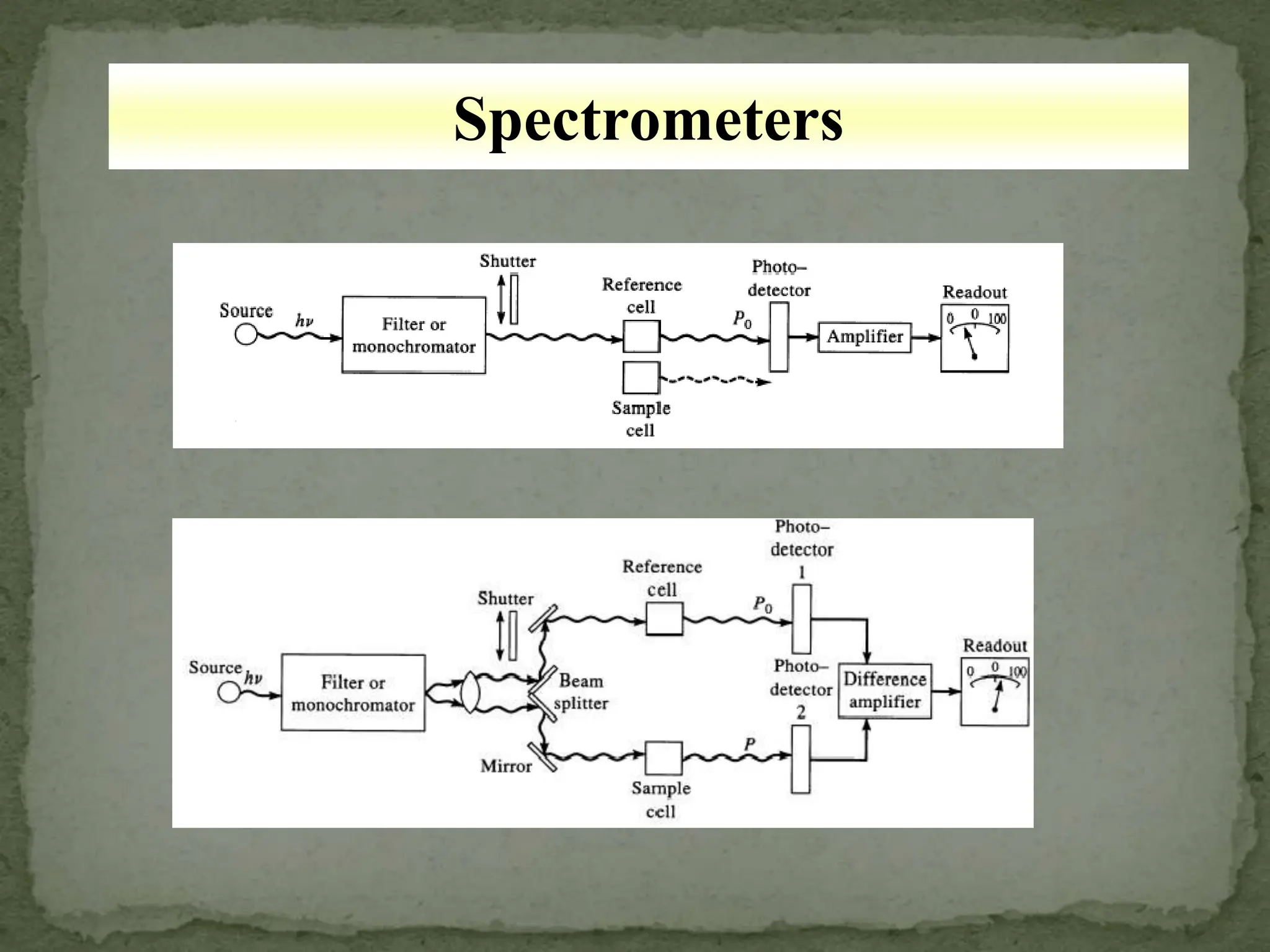
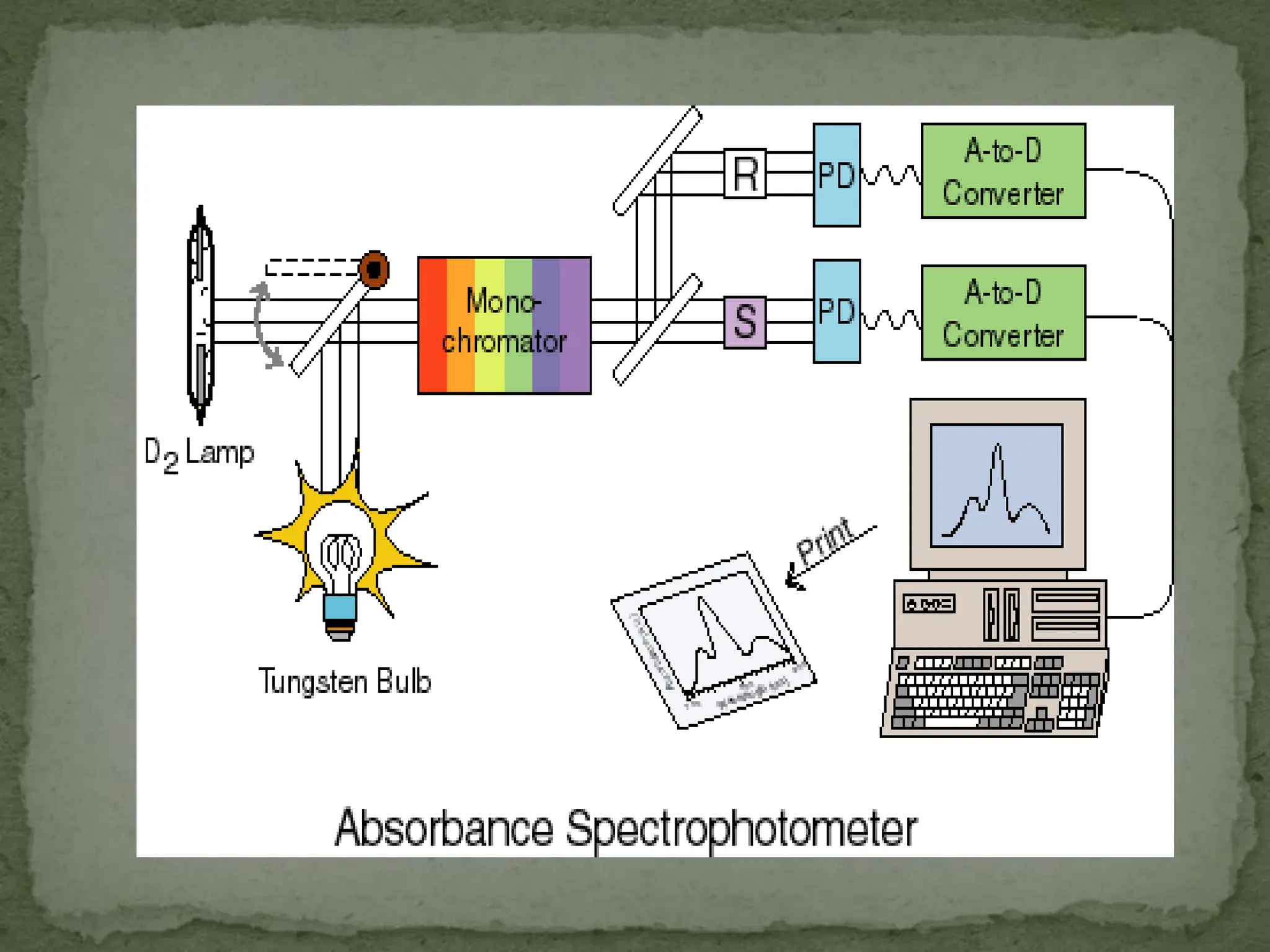
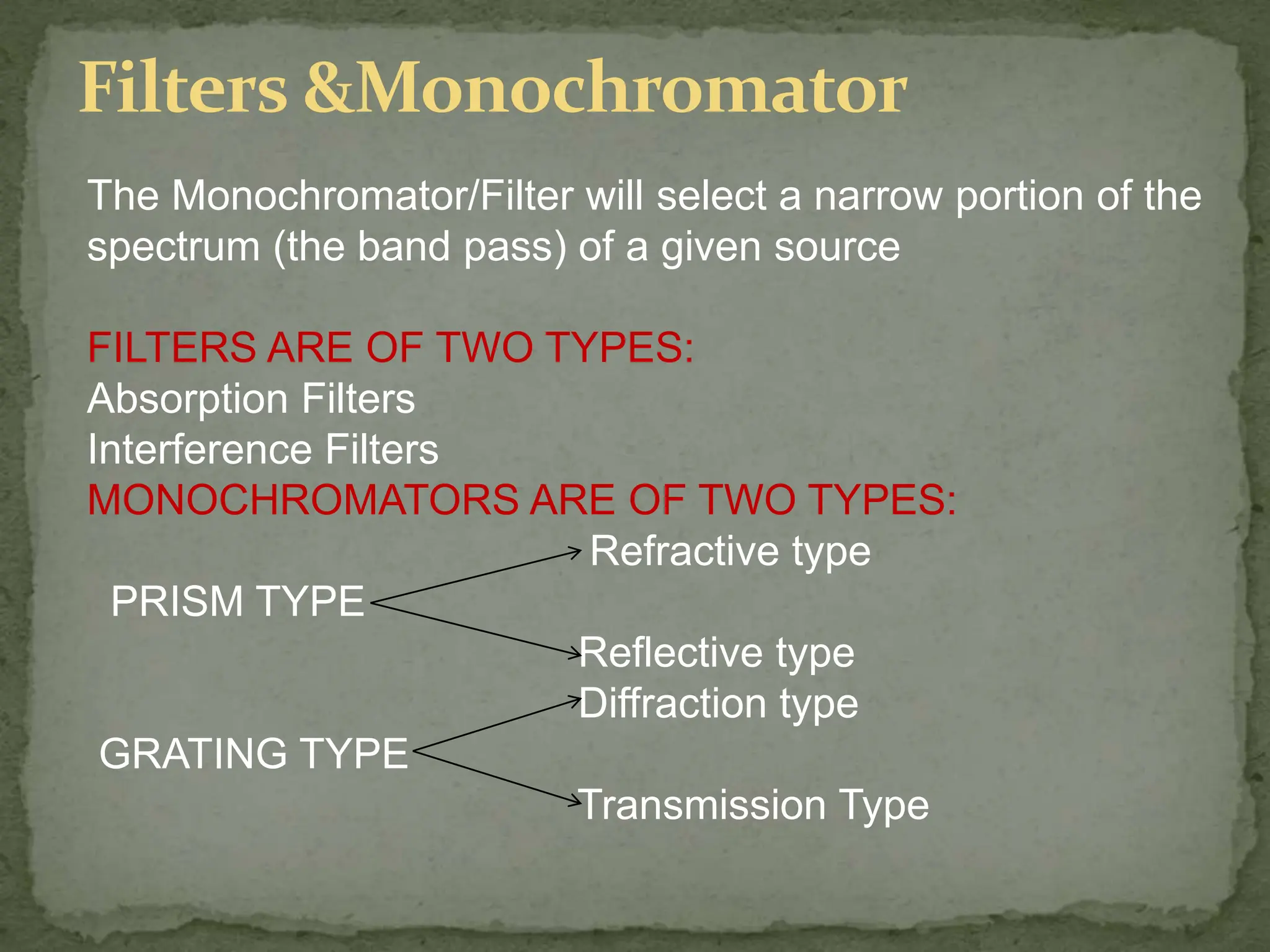
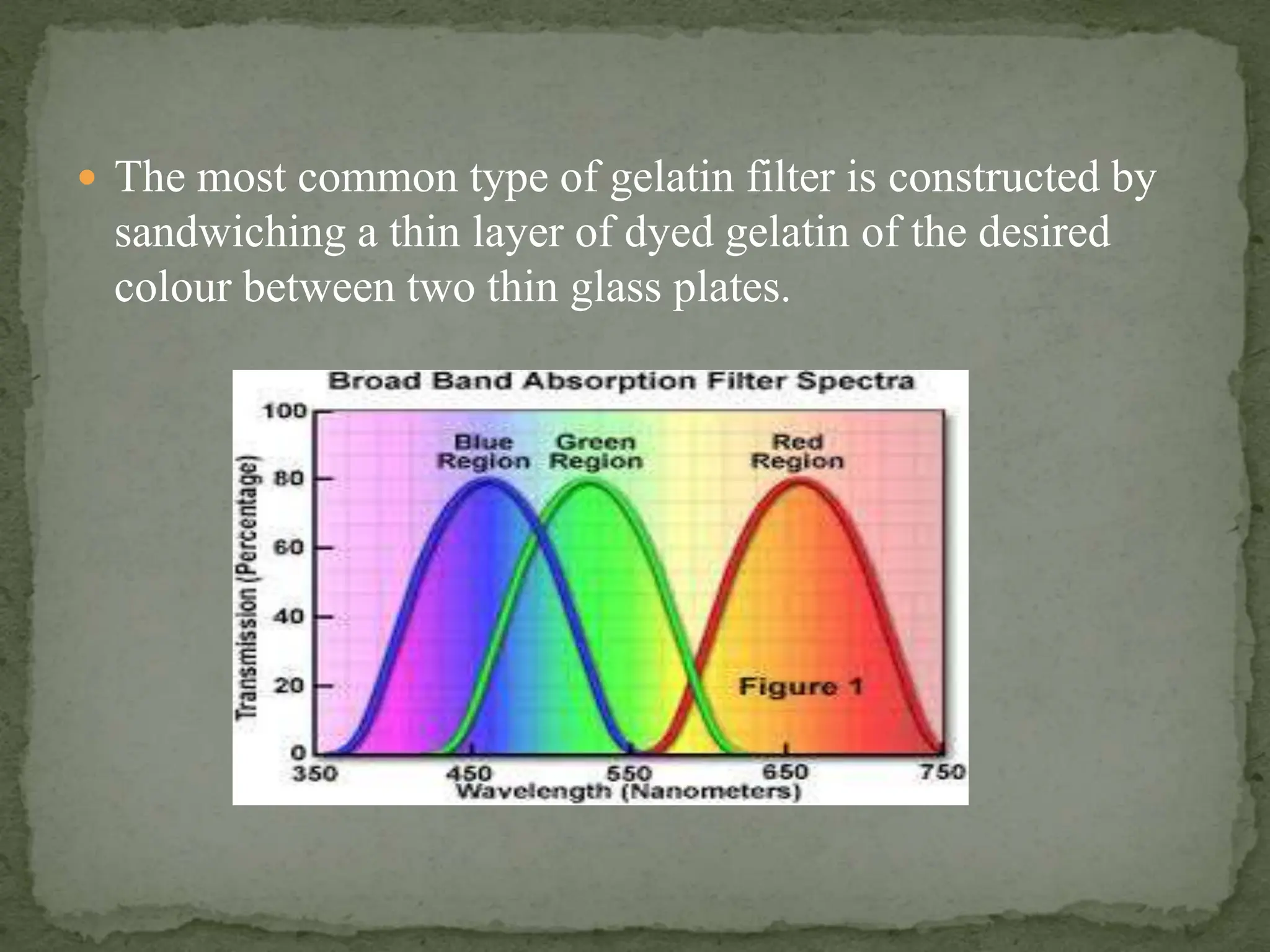
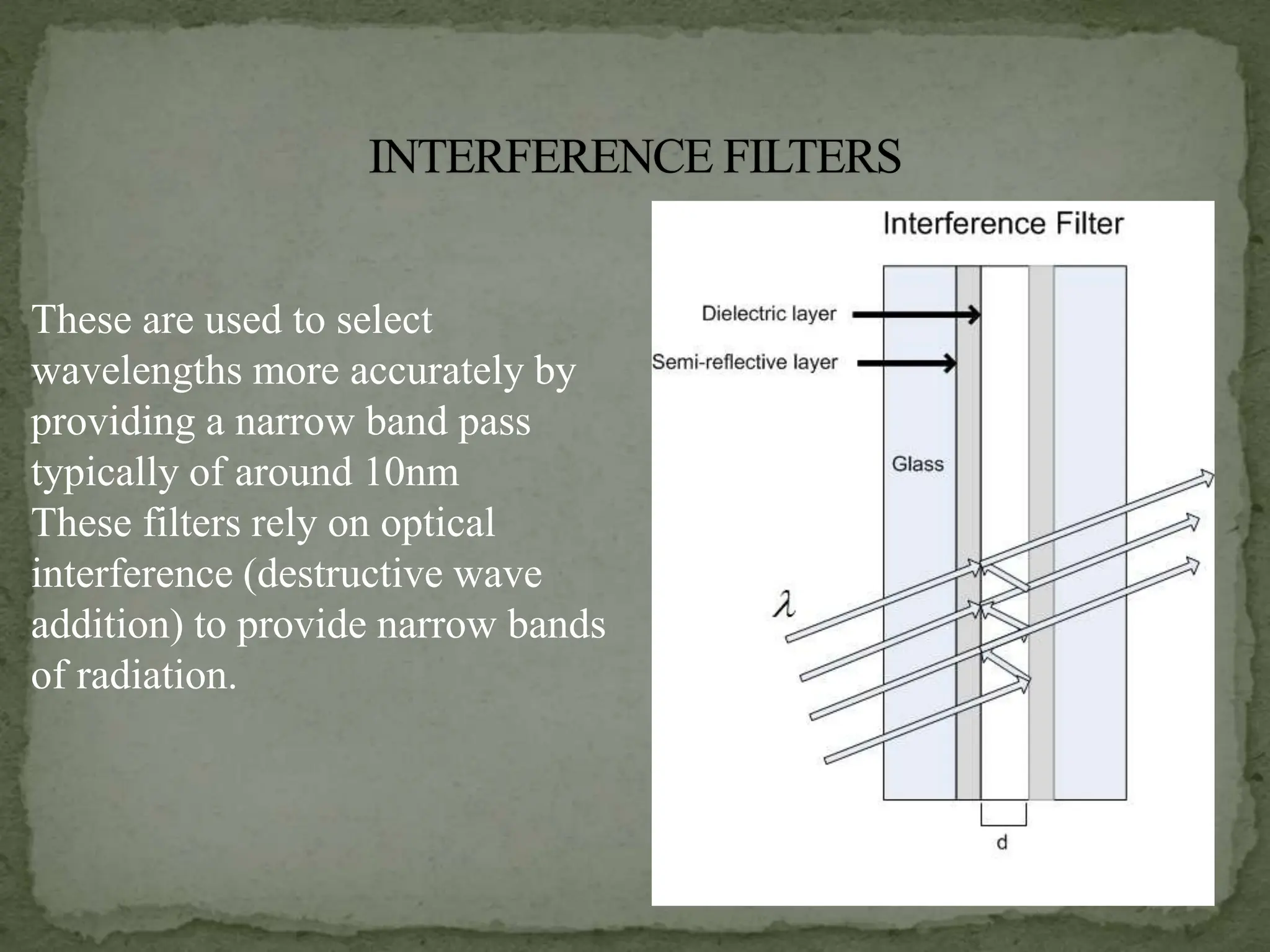
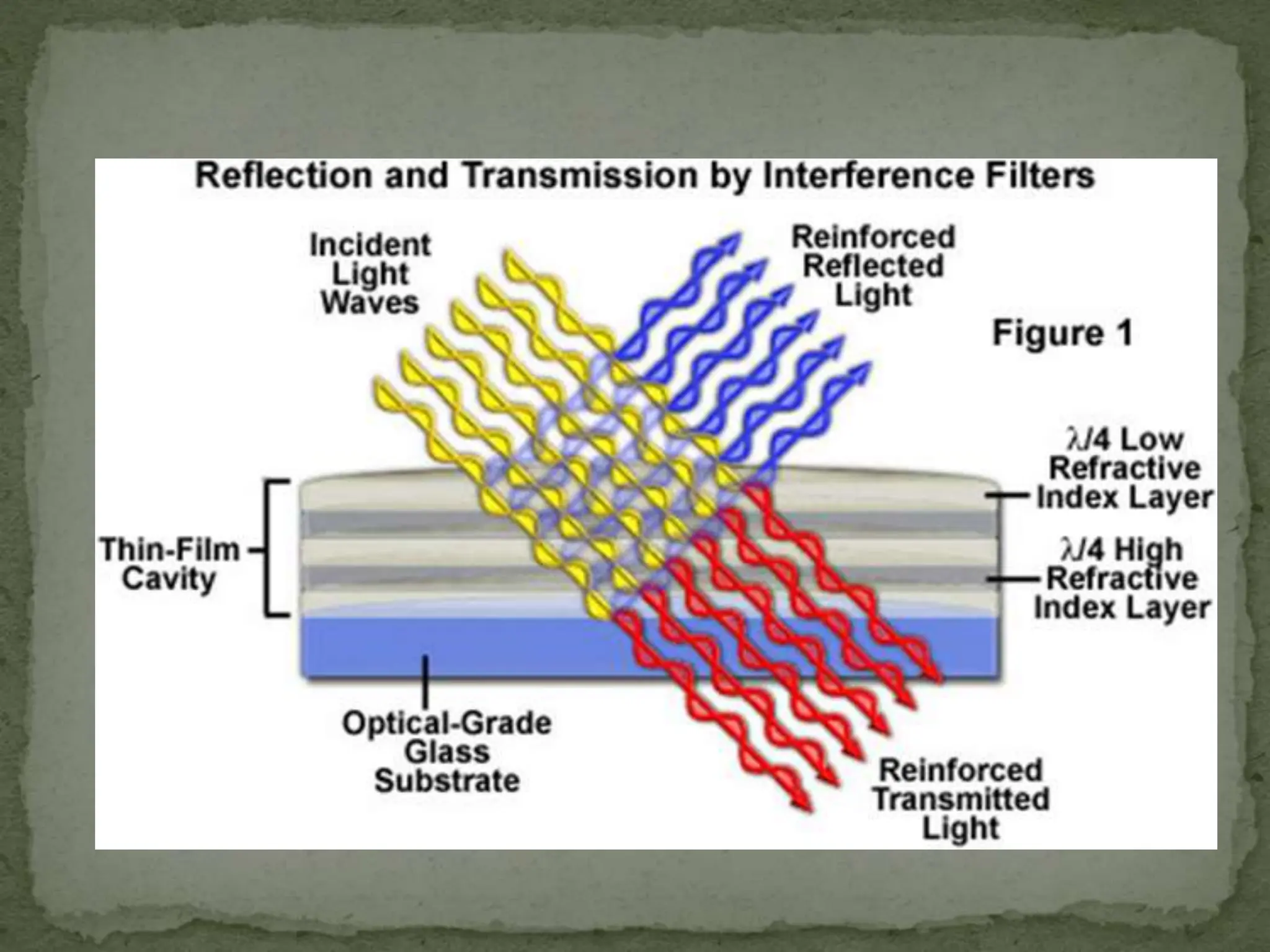
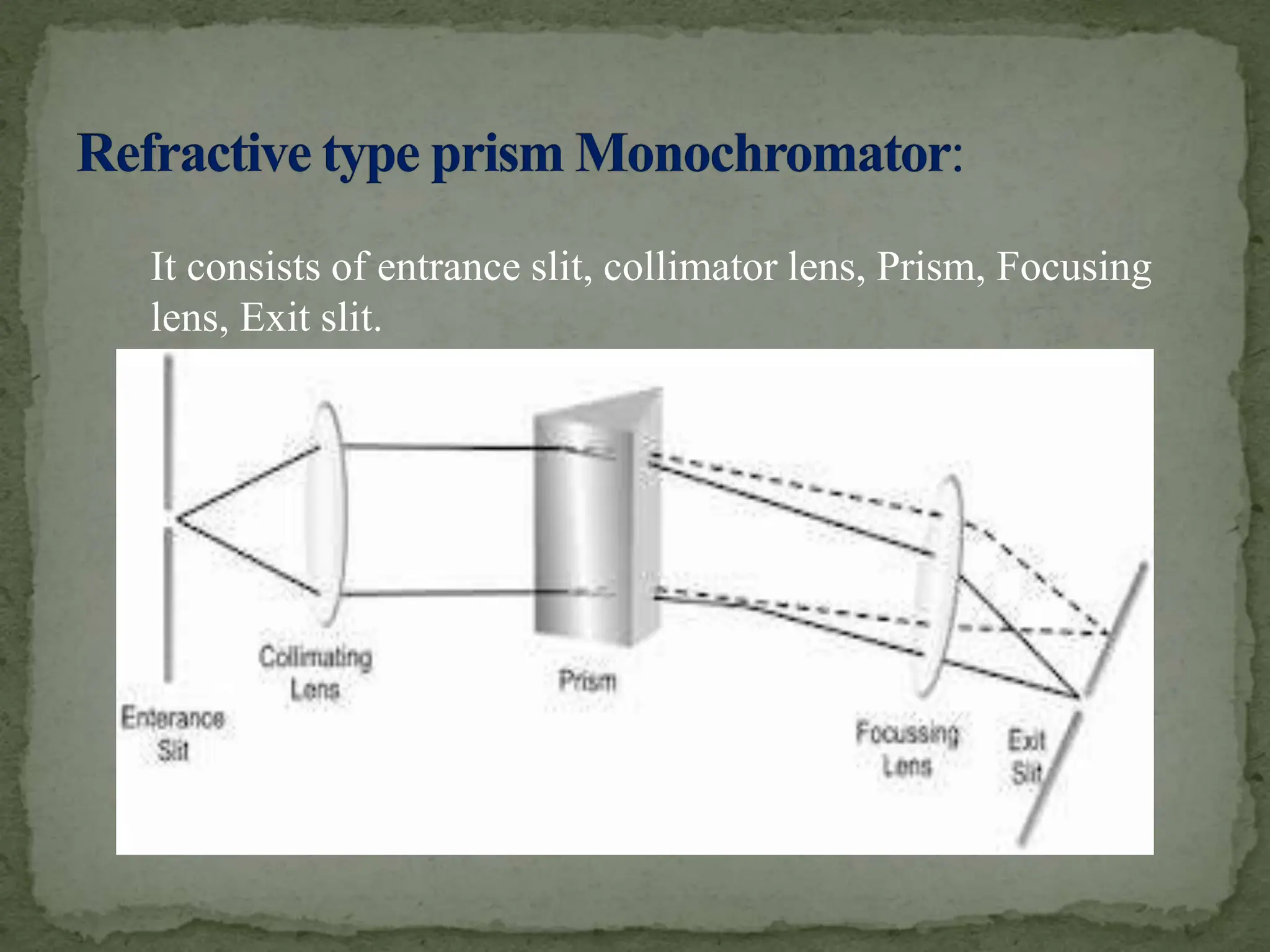
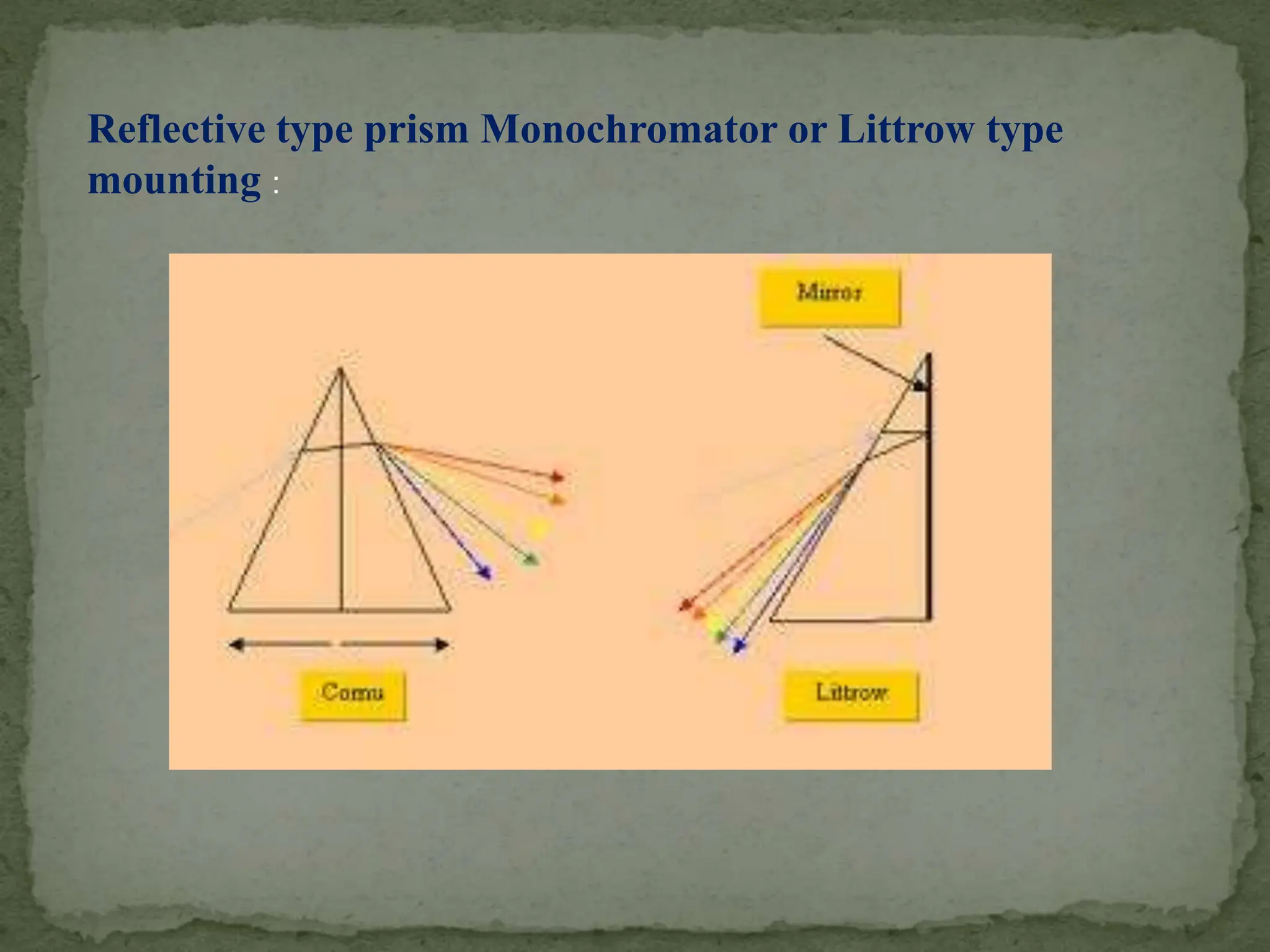
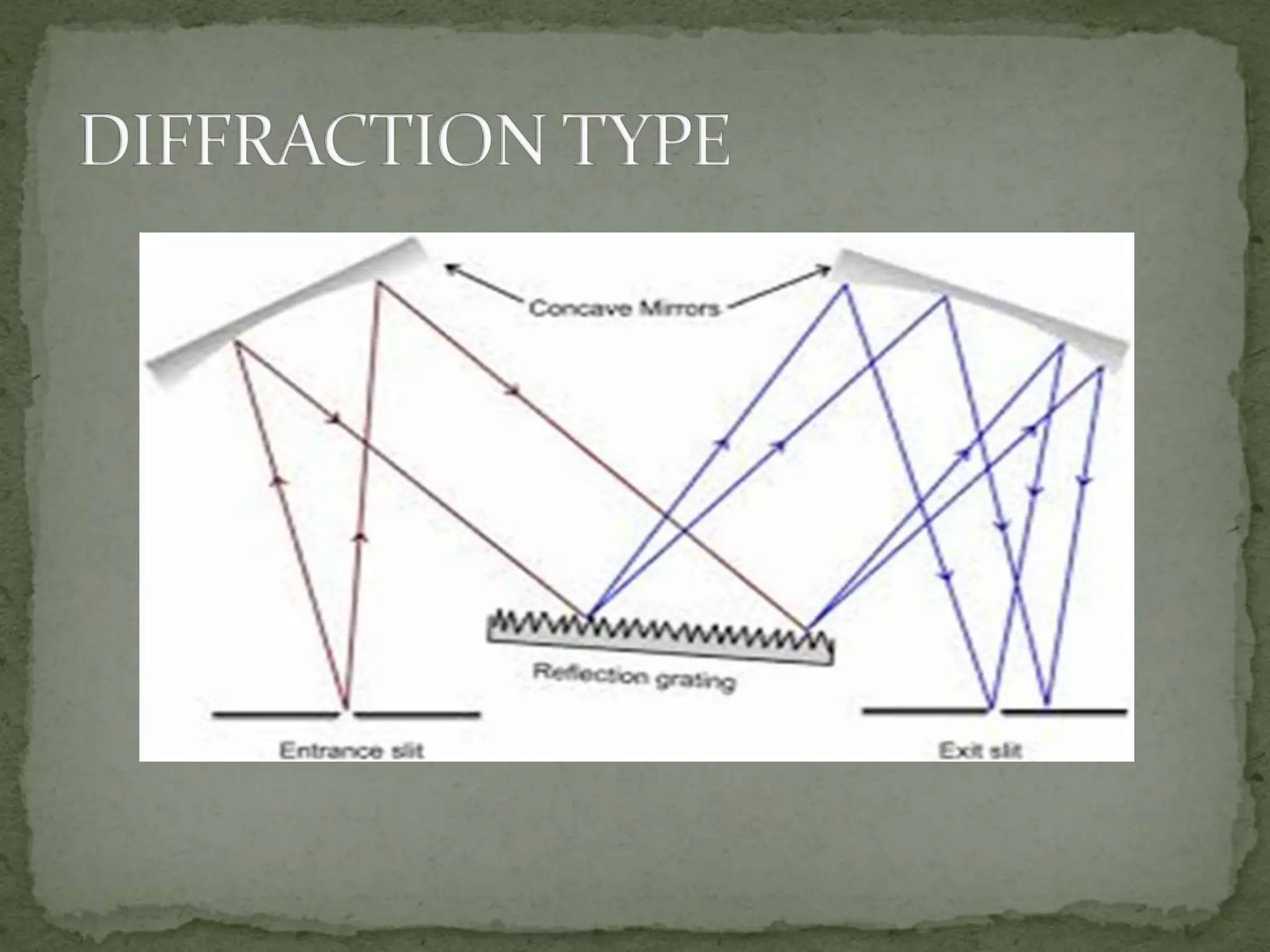
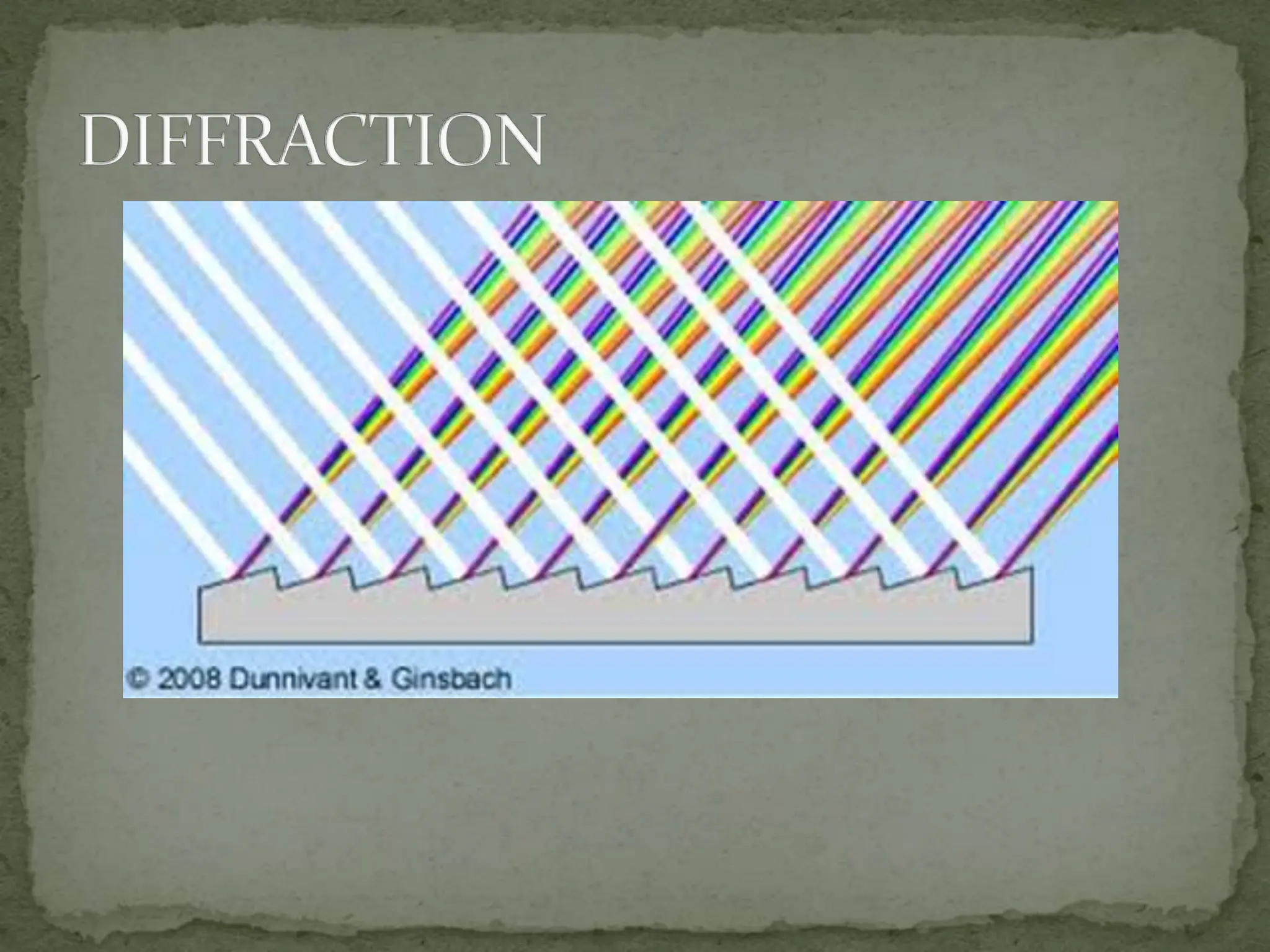
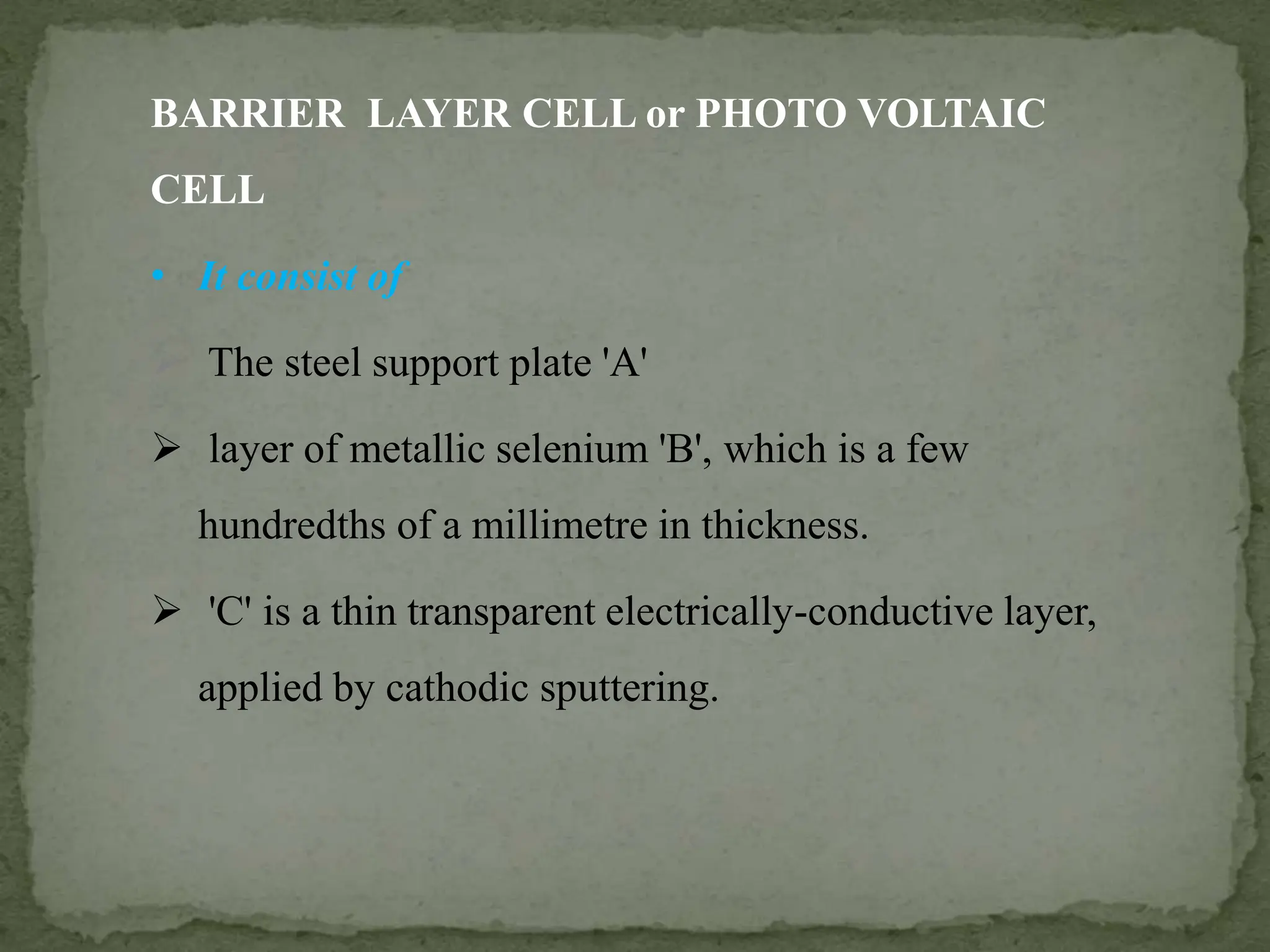
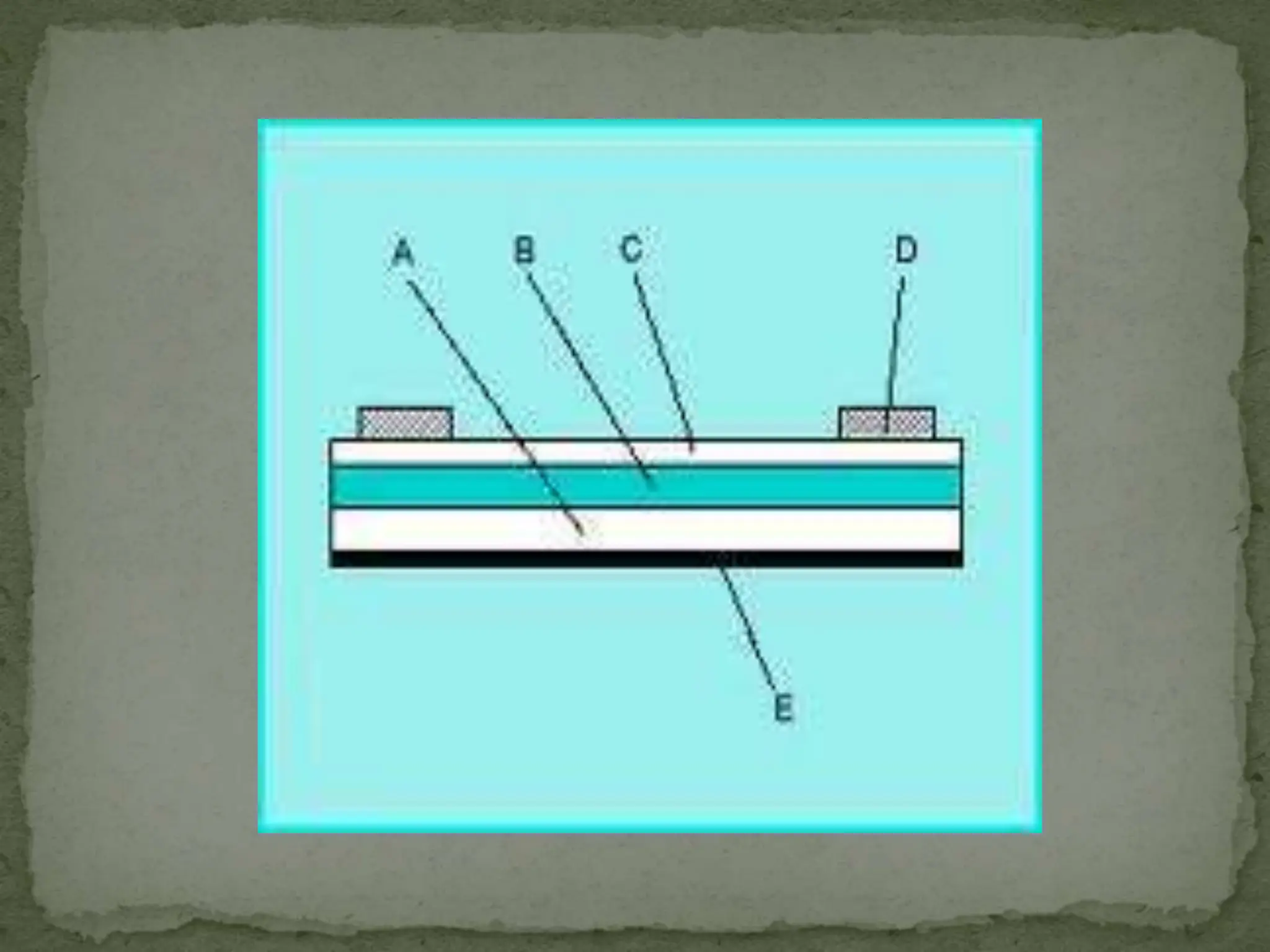
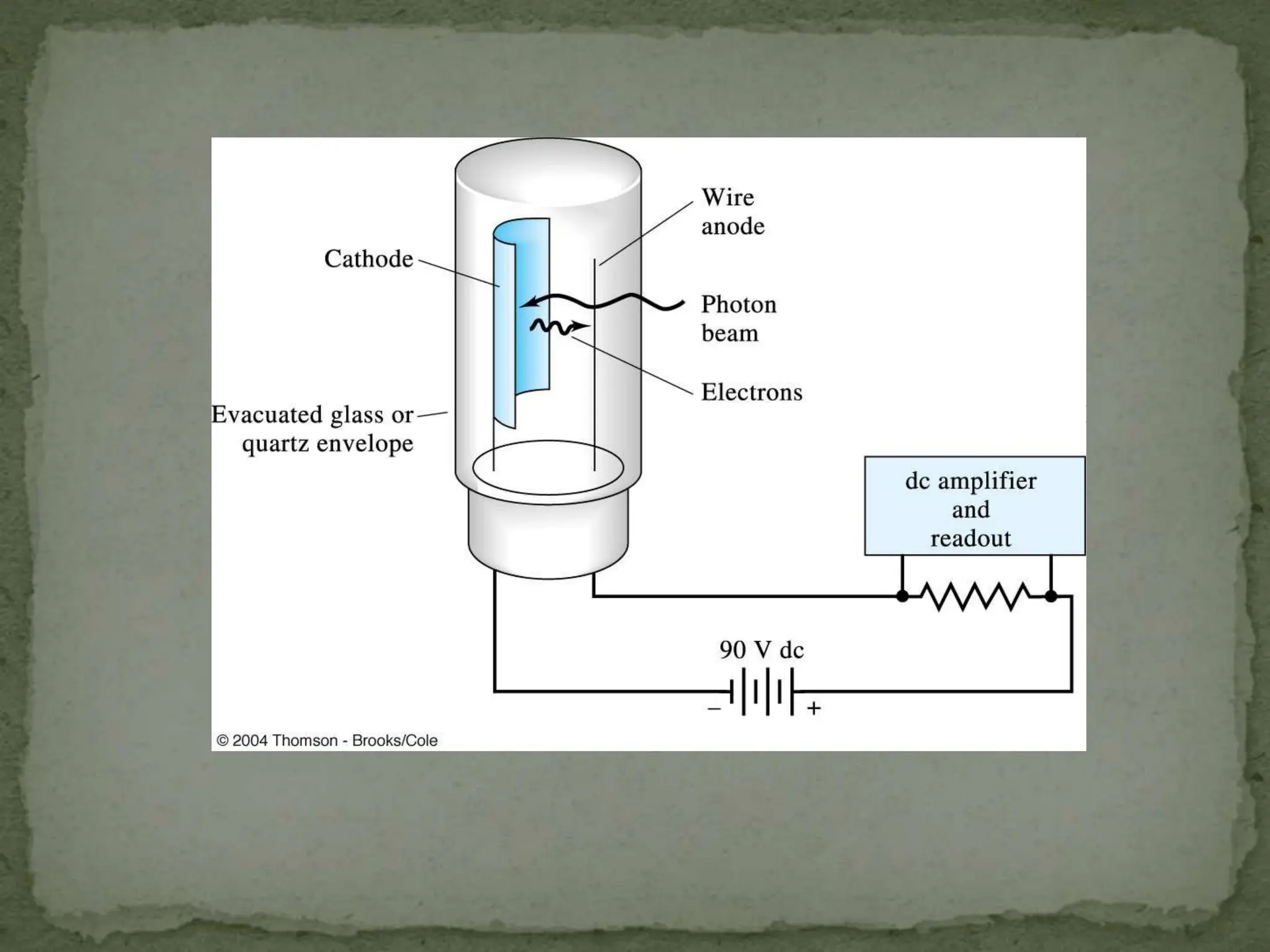
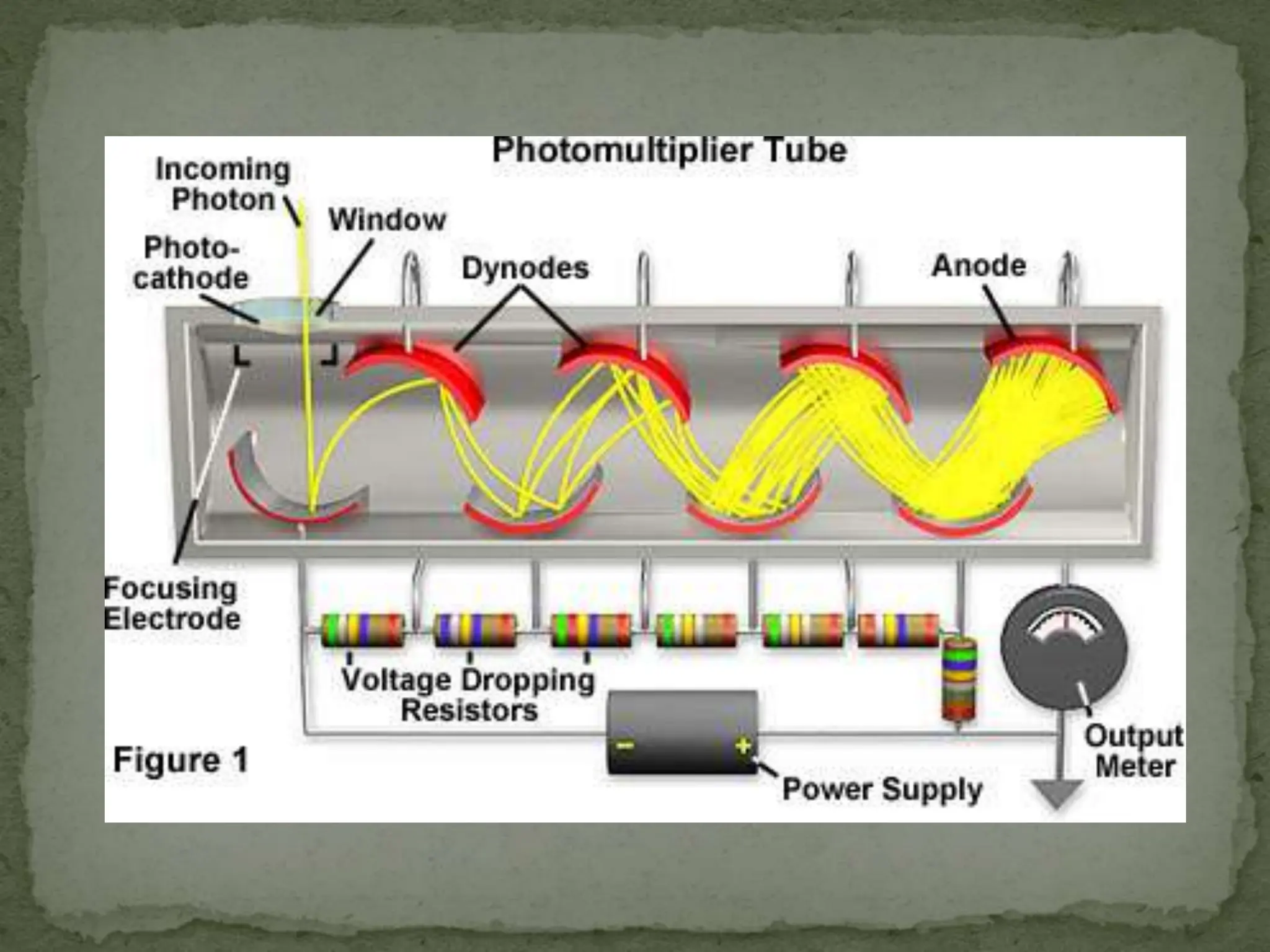
Light interacting with matter as an analytical tool
The document discusses the use of electromagnetic radiation across the electromagnetic spectrum as an analytical tool when interacting with matter. It covers topics like the electromagnetic spectrum, light behaving as both a wave and particle, spectroscopy terminology, various light sources, components of spectrometers like monochromators, sample compartments, and detectors. It also discusses applications of spectroscopy in qualitative and quantitative analysis of organic and inorganic compounds.