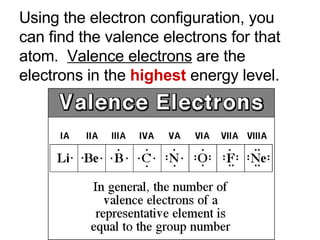
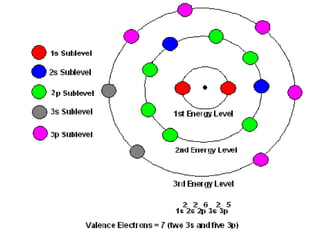
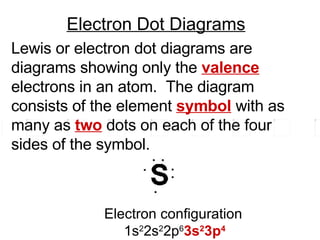
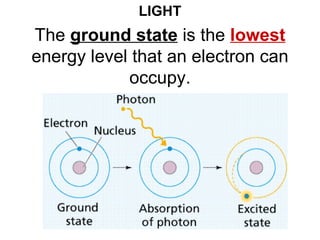
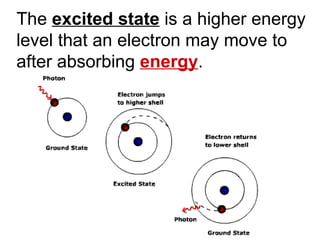
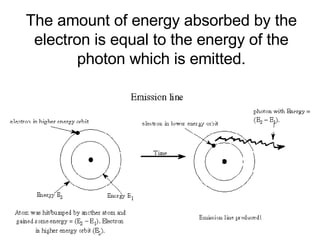
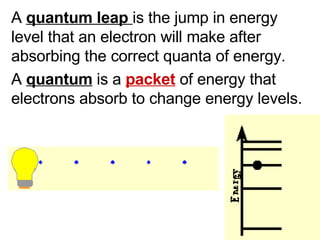
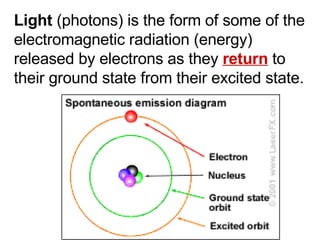
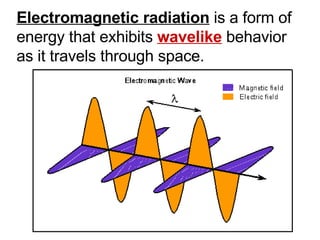
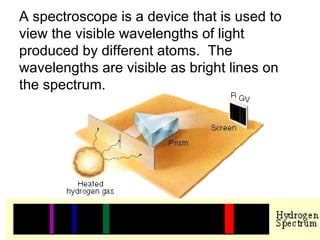
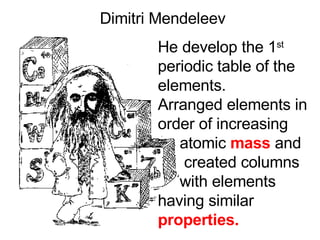
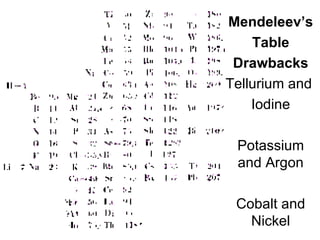
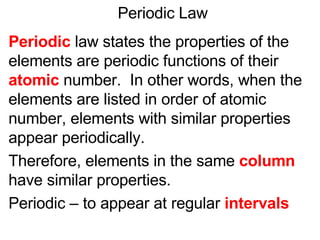
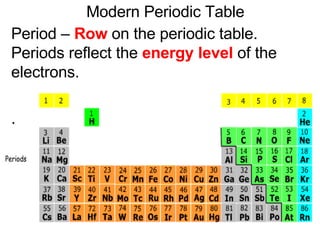
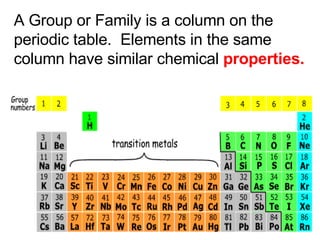
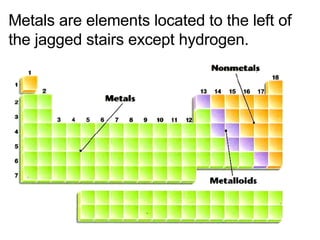
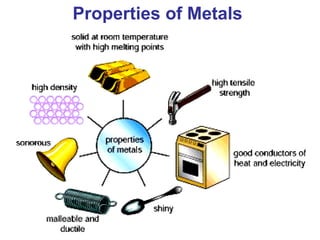
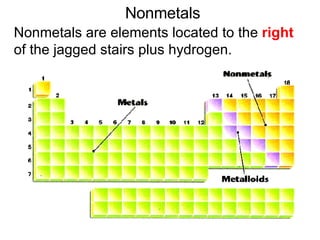
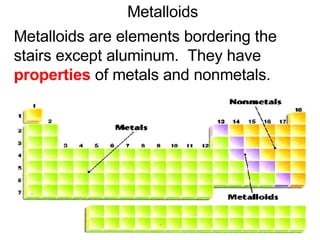
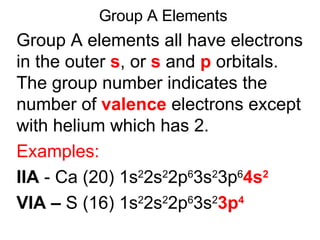
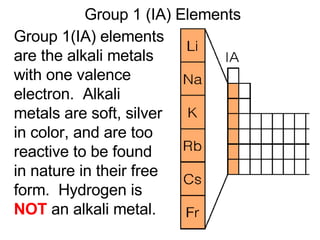
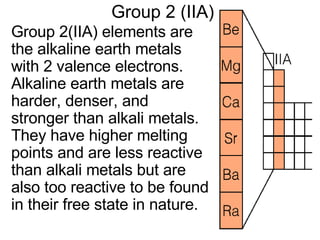
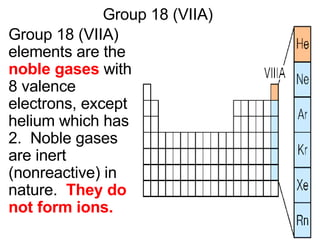
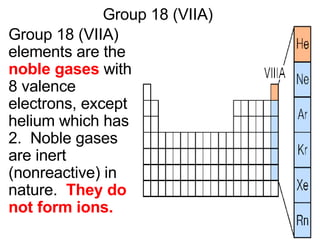
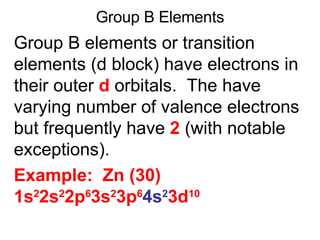
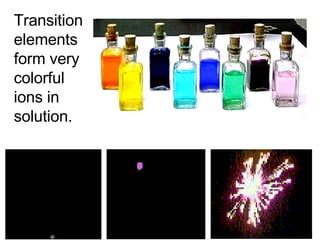
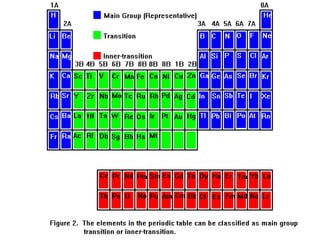
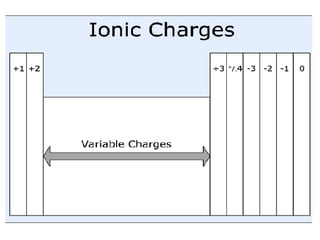
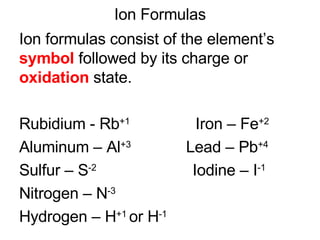
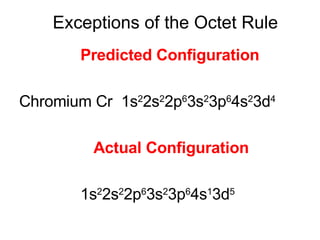
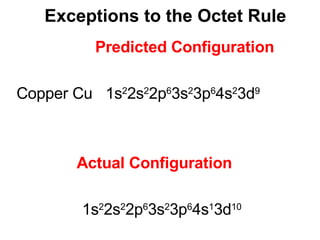
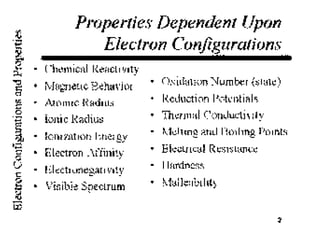
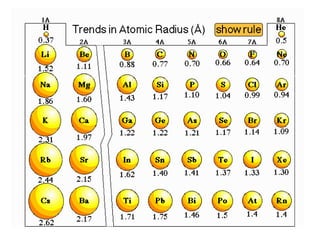
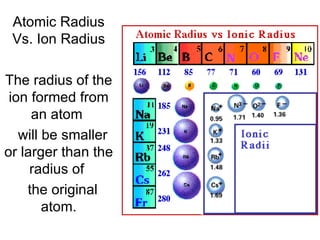
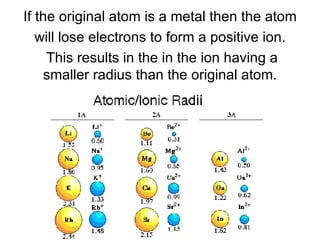
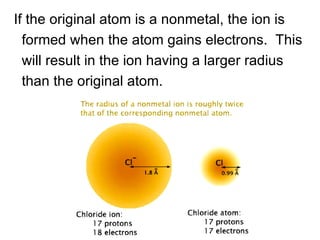
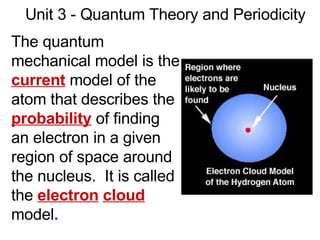
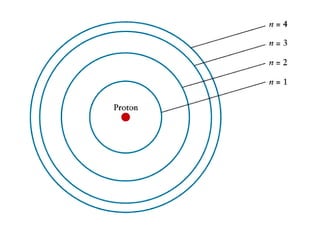
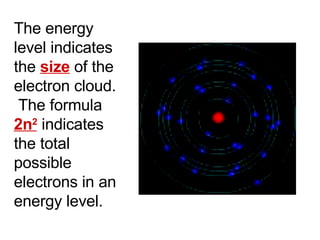
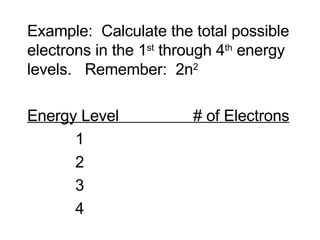
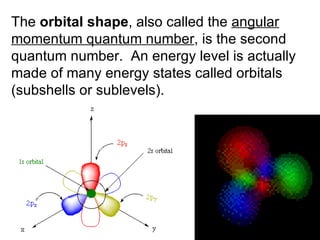
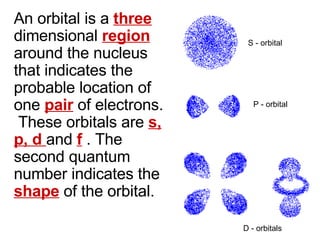
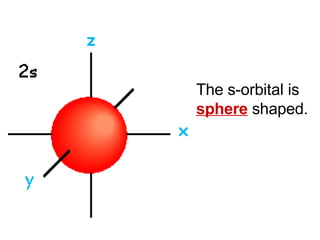
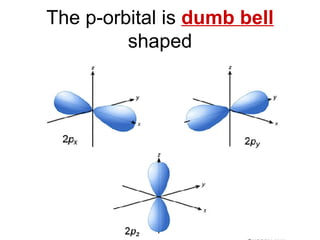
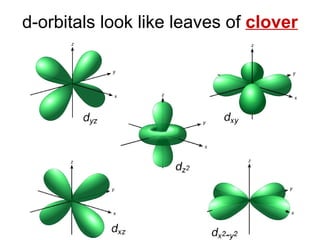
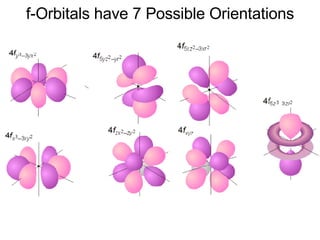
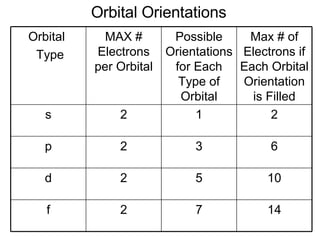
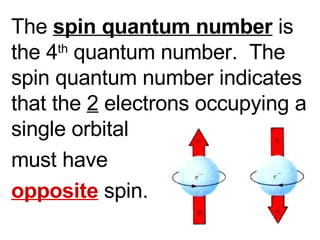
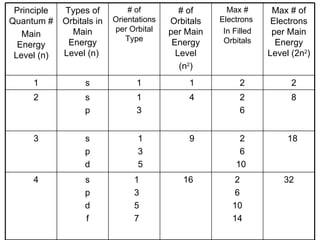
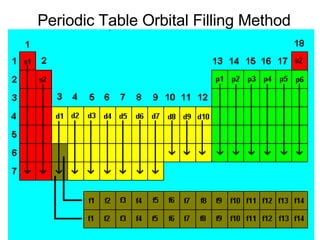
This document provides an overview of quantum theory and periodicity in chemistry. It discusses the quantum mechanical model of the atom, quantum numbers, electron configurations, orbital shapes and orientations. It also covers periodic trends, ion formation, exceptions to predicted configurations, and periodic properties. Key topics include the electron cloud model, principal and angular momentum quantum numbers, s, p, d and f orbitals, writing configurations, ion charges, and how periodic trends relate to electron configurations.


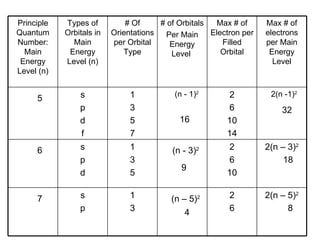
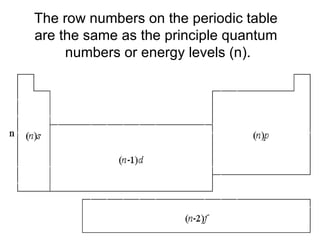
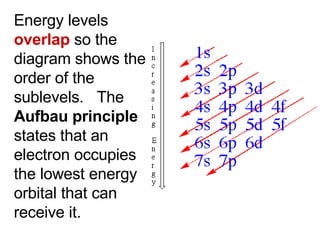
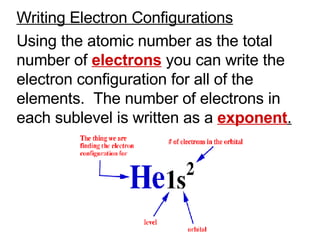


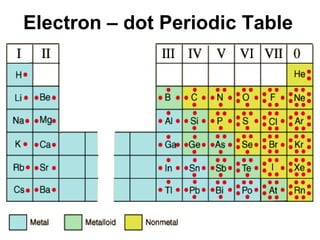
![Noble – Gas Notation The Group VIII elements, helium, argon, krypton, xenon, and radon are called the noble gases. The configurations of the noble gases are often used as a shorthand method for writing longer electron configurations. For Example: Sodium – Na has 11 electrons Electron Configuration 1s 2 2s 2 2p 6 3s 1 Noble – Gas Configuration [Ne]1s 2](https://image.slidesharecdn.com/unit3presentation4201/85/Unit3presentation-29-320.jpg)
![Example 2: Arsenic, As, 33 electrons Electron Configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 3 Noble – Gas Configuration [Ar]4s 2 3d 10 4p 3 Example 3: Barium, Ba, 56 electrons Example 4: Rubidium, Rb](https://image.slidesharecdn.com/unit3presentation4201/85/Unit3presentation-30-320.jpg)