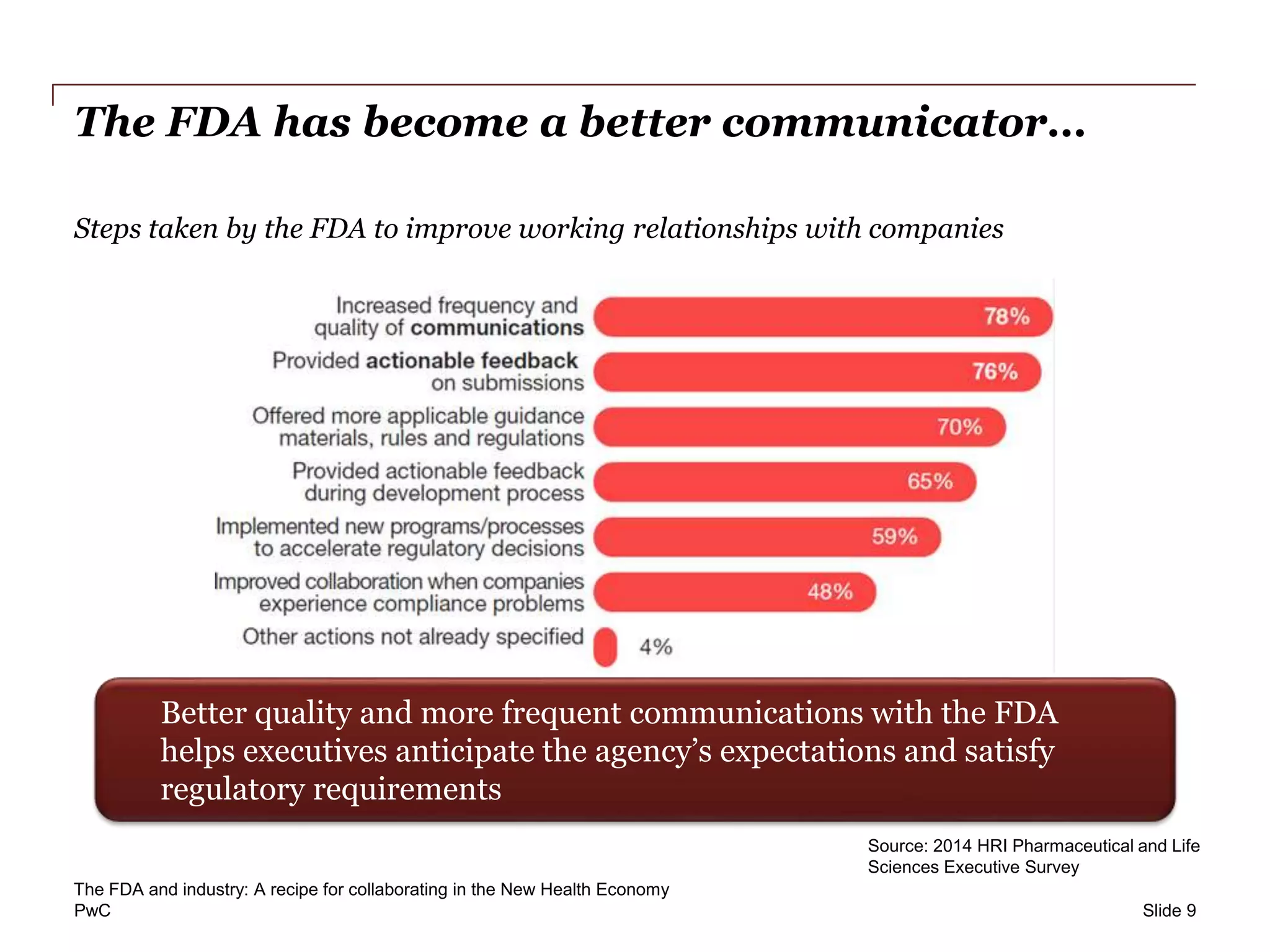
This report from PwC examines the evolving relationship between the FDA and the pharmaceutical industry, highlighting the need for improved communication and a collaborative approach to innovation and regulatory processes. A survey of industry executives and consumers reveals a desire for greater engagement and understanding of value in drug therapies, while acknowledging the challenges posed by user fees and regulatory timelines. The document emphasizes the importance of consumer input and suggests future strategies for minimizing risk and accelerating access to experimental therapies.