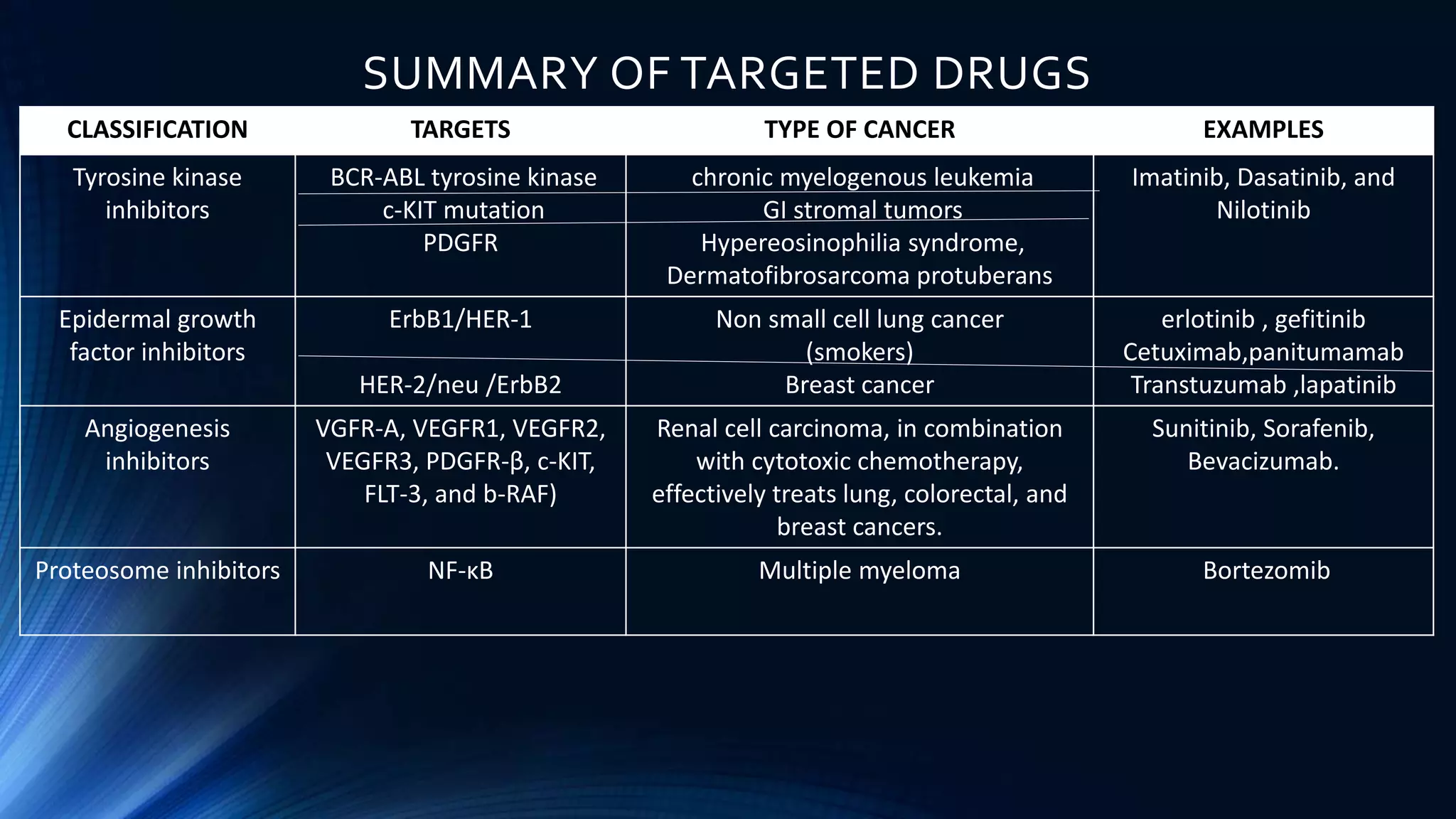
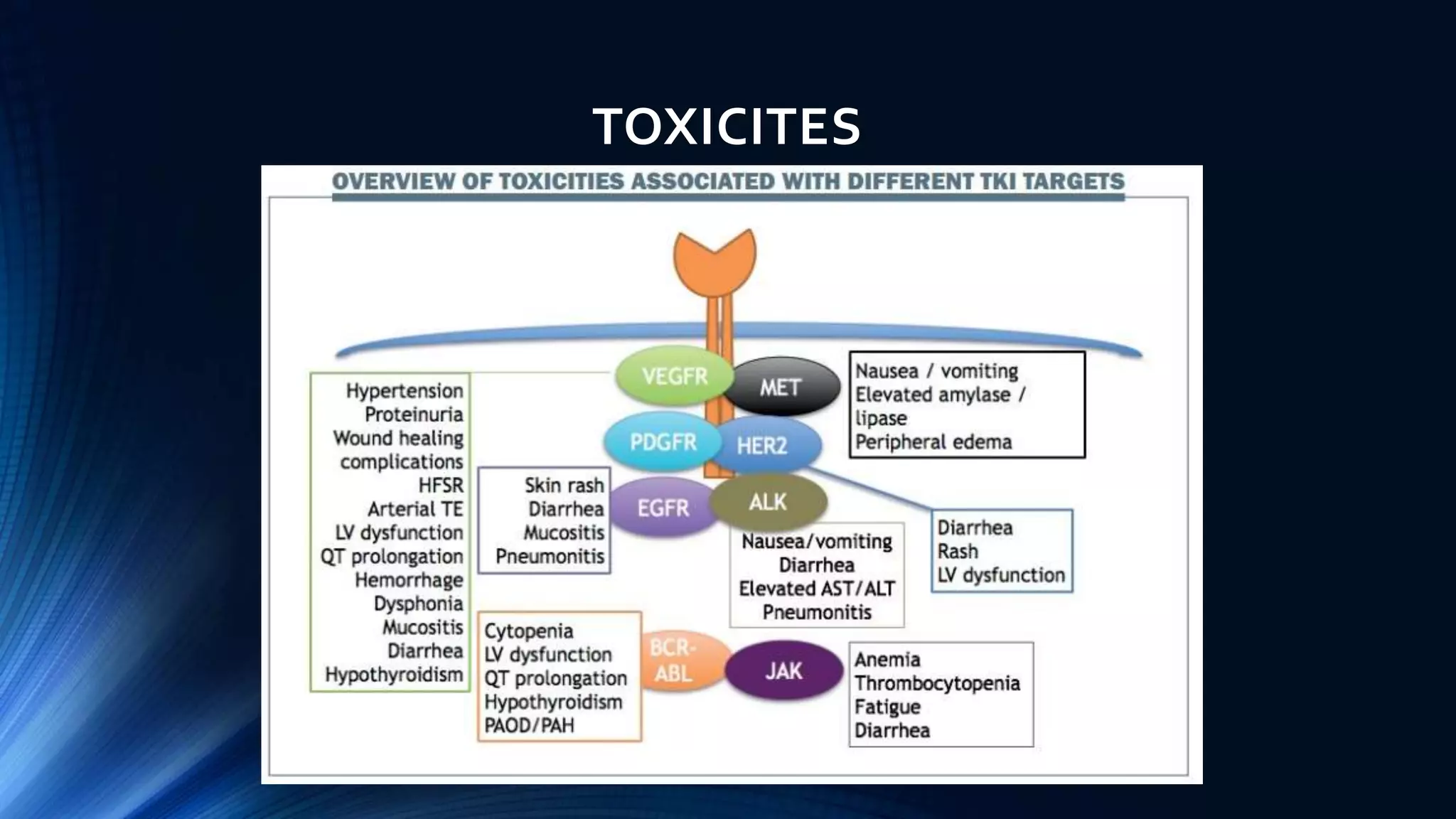
This document discusses targeted cancer therapies and their mechanisms of action. It outlines 10 hallmarks of cancer and describes targeted drugs that inhibit specific proteins and pathways involved in cancer growth. These targeted drugs include small molecule tyrosine kinase inhibitors, monoclonal antibodies, angiogenesis inhibitors, and proteosome inhibitors. Examples are provided of targeted therapies used to treat cancers like chronic myeloid leukemia, lung cancer, breast cancer, and multiple myeloma. Potential side effects of targeted therapies are also mentioned.