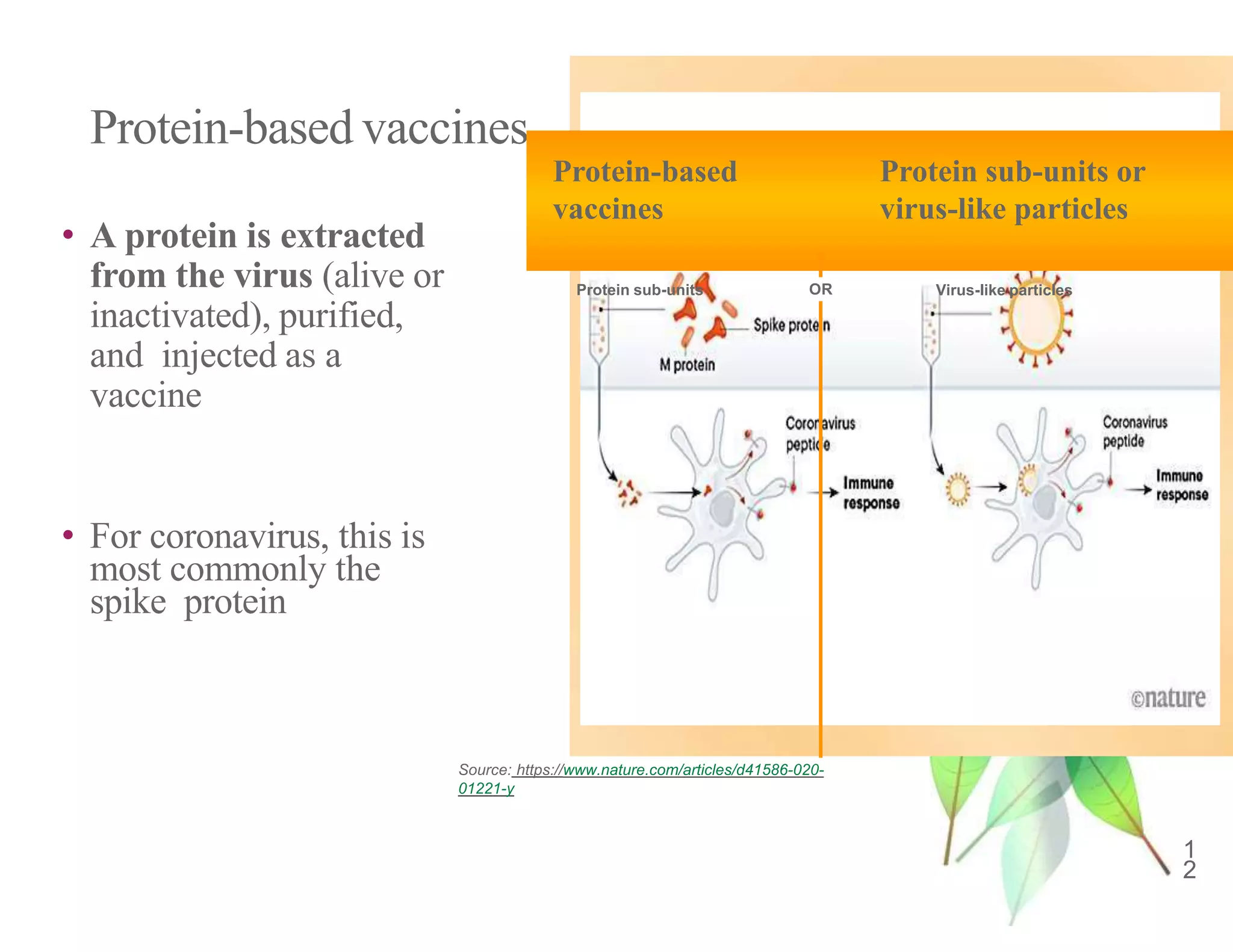
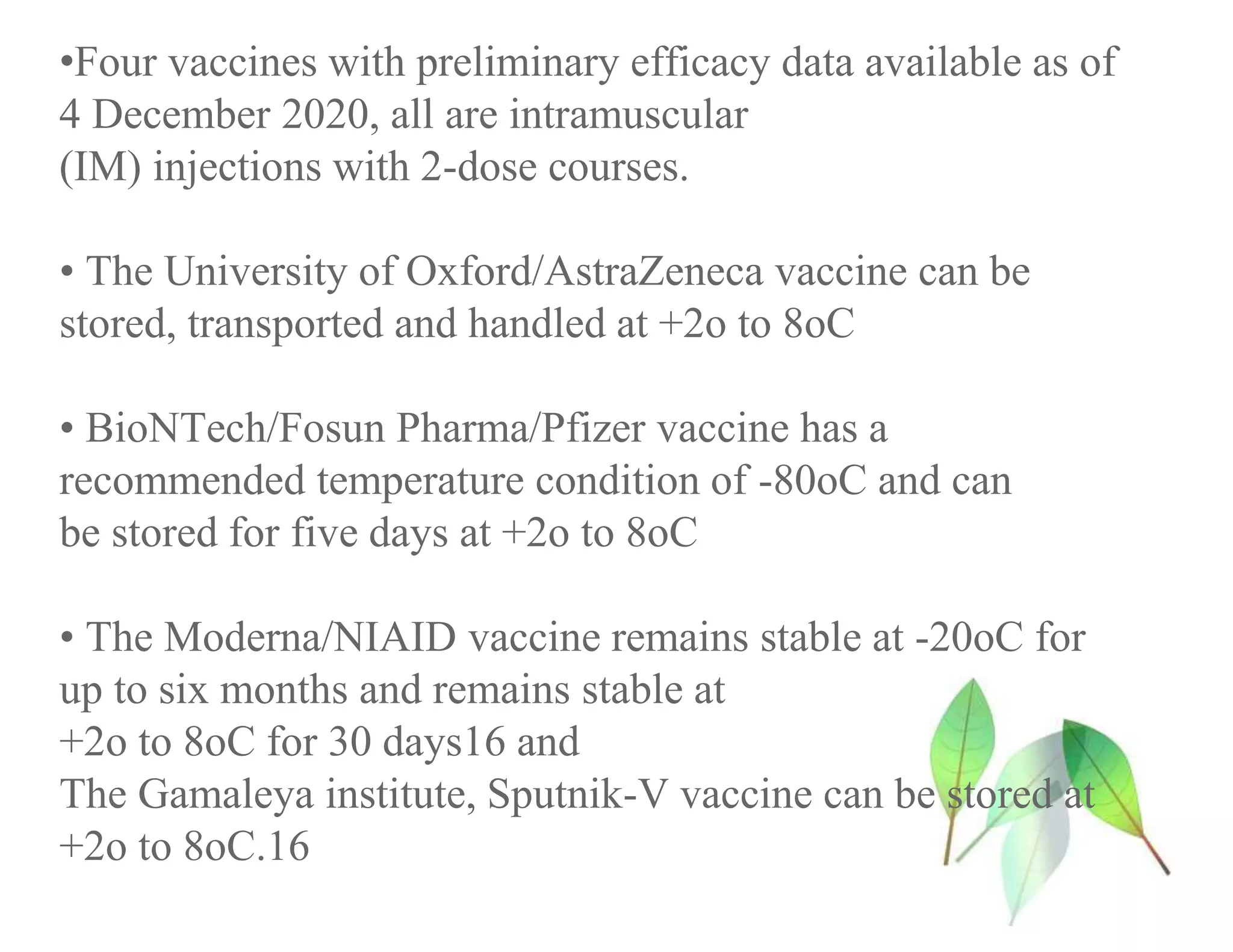
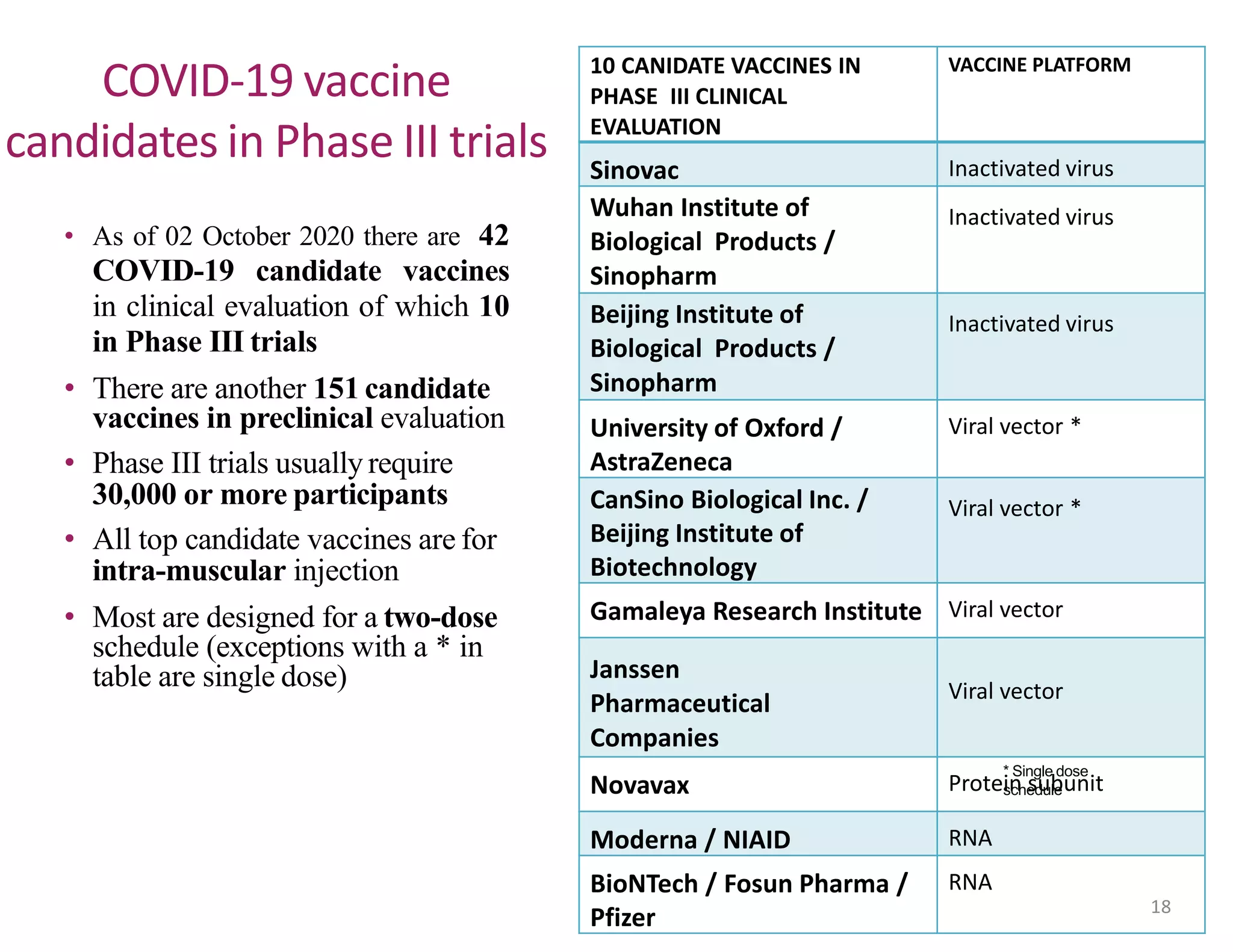
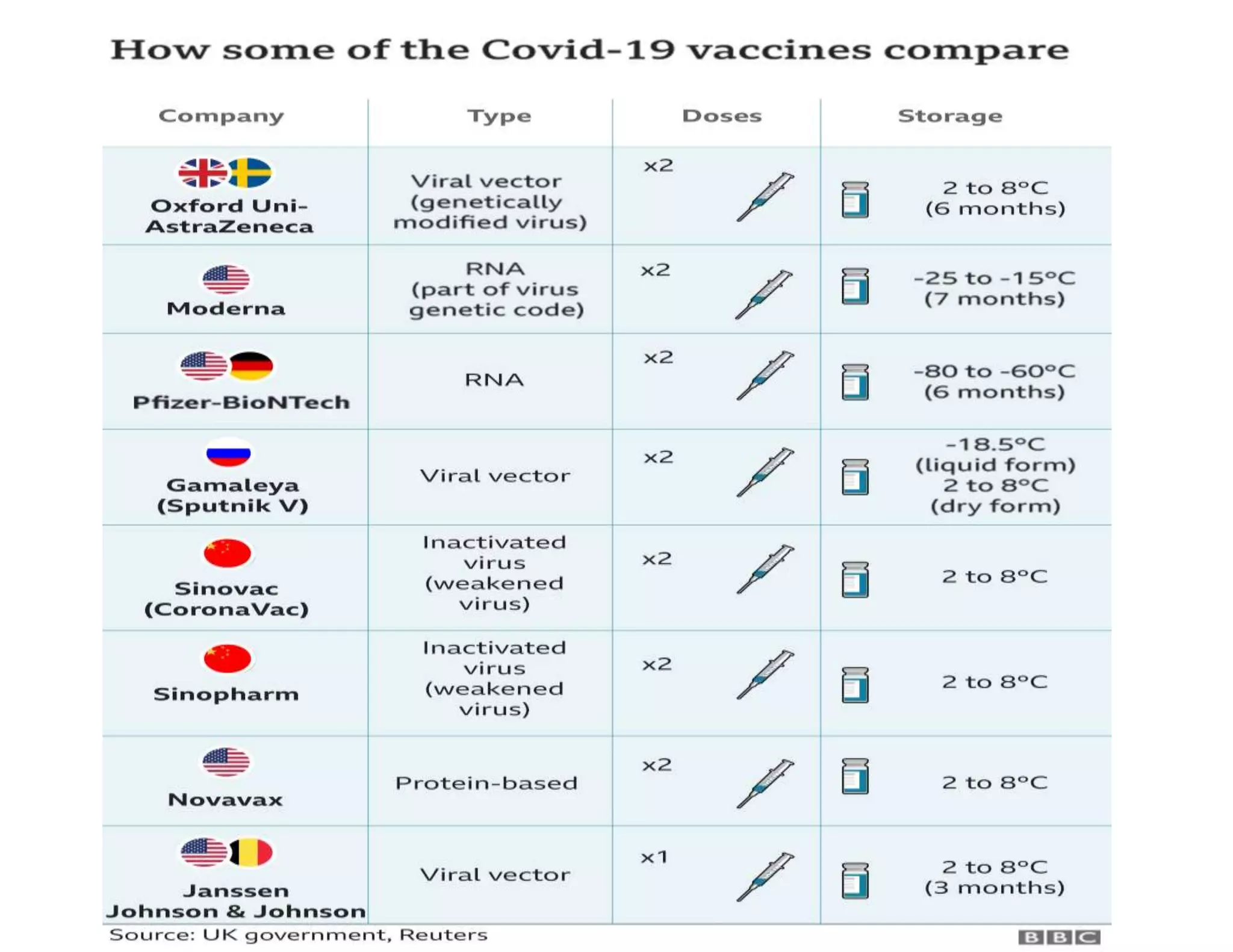
The document discusses COVID-19 vaccine development and types. It explains that vaccines work by training the immune system to recognize pathogens through immunogens like attenuated live viruses, inactivated viruses, viral proteins, or genetic material. For COVID-19, vaccines in late-stage trials use inactivated viruses, viral vectors, protein subunits, and RNA or DNA. The development process involves pre-clinical and clinical trials through phase III. As of October 2020, 10 candidates were in phase III worldwide trials to evaluate safety and efficacy.