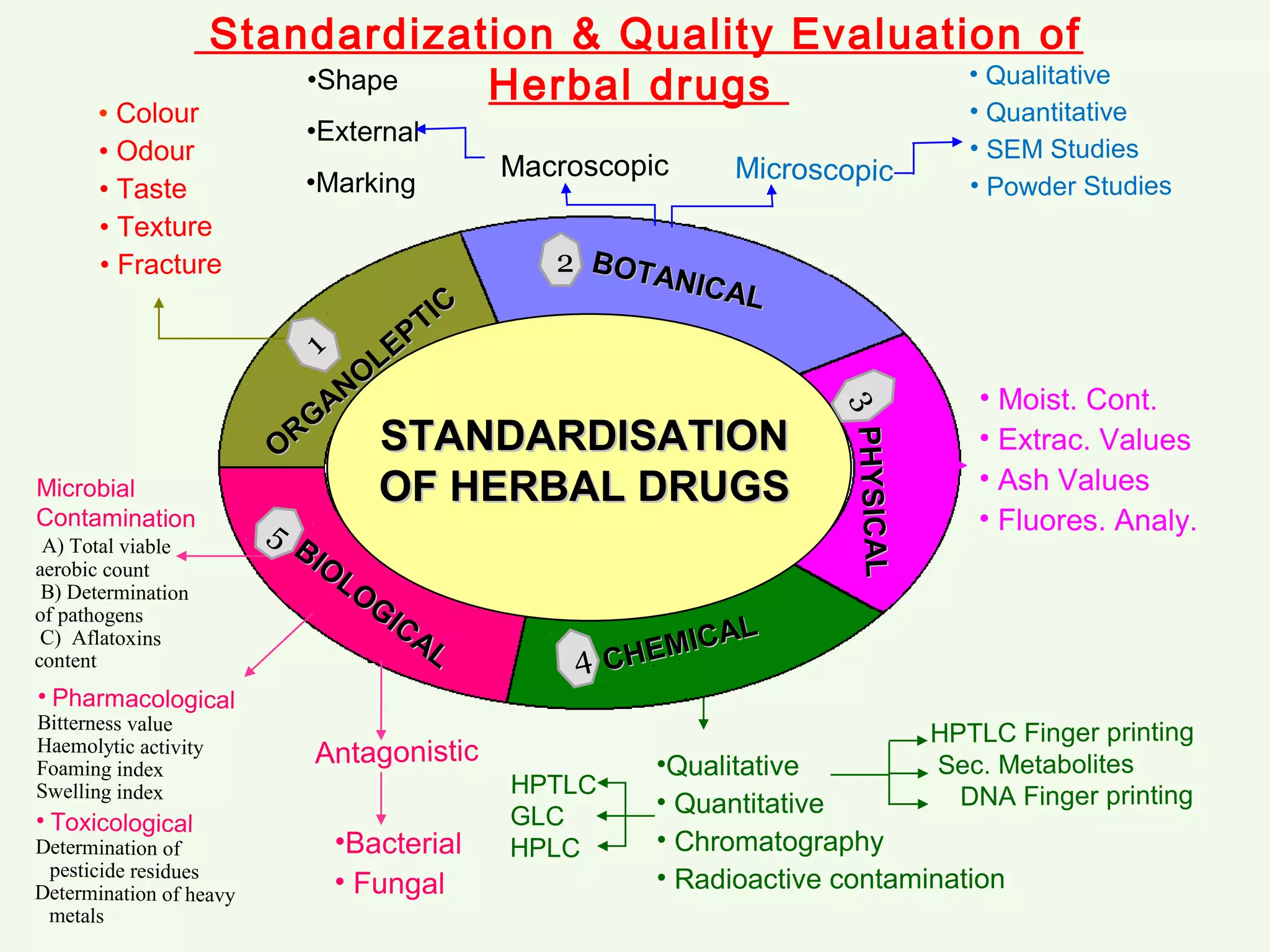
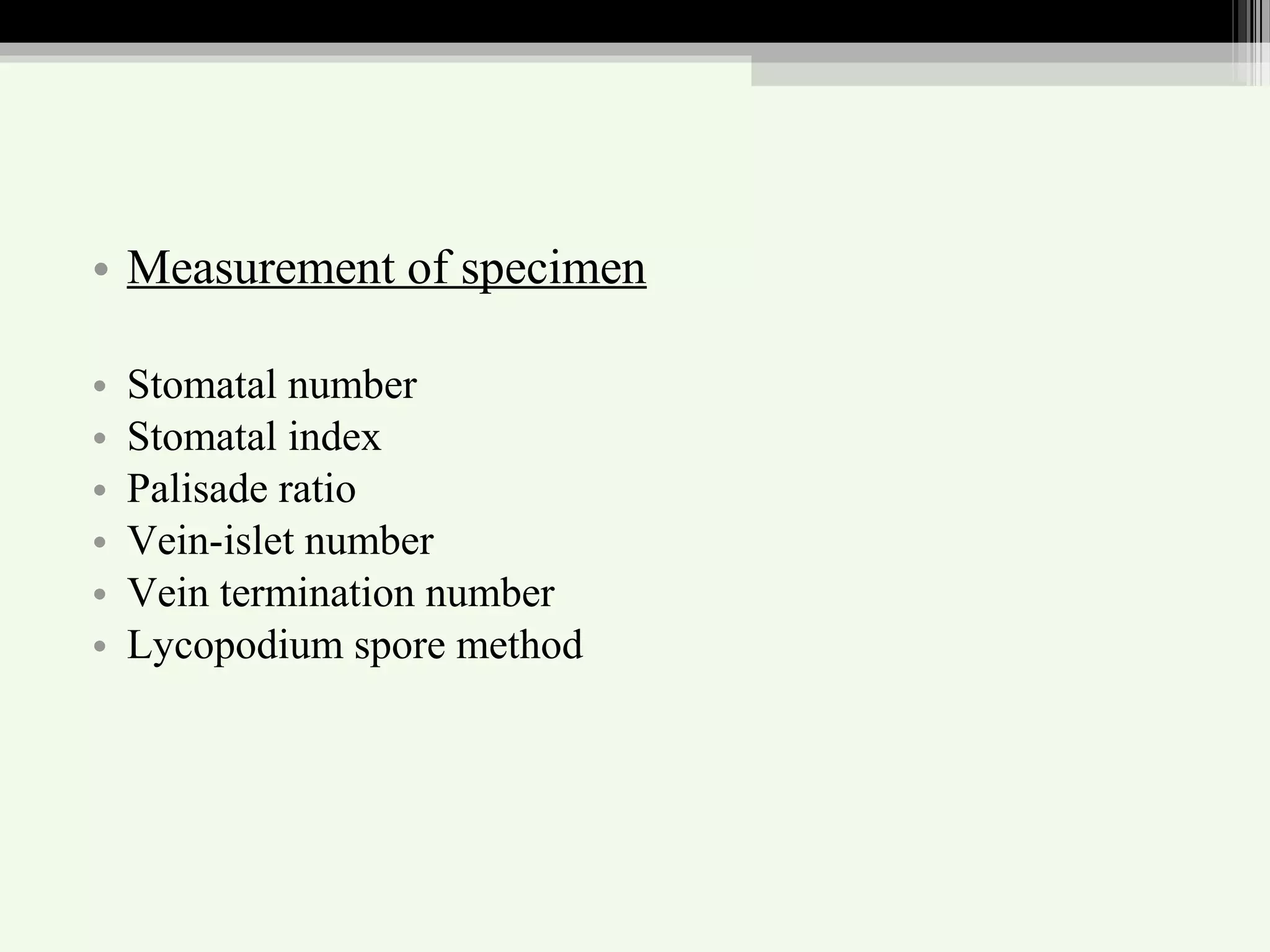
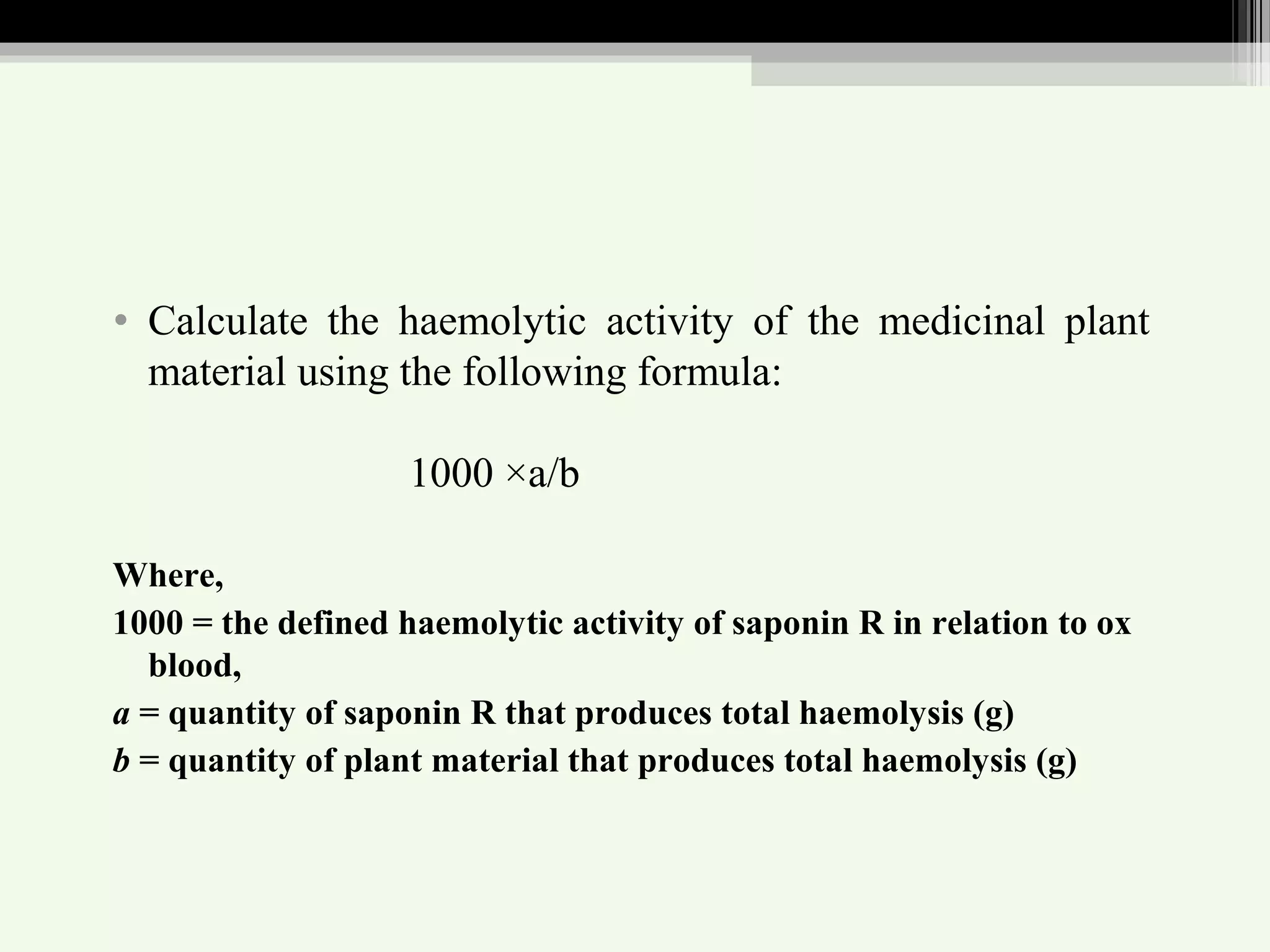
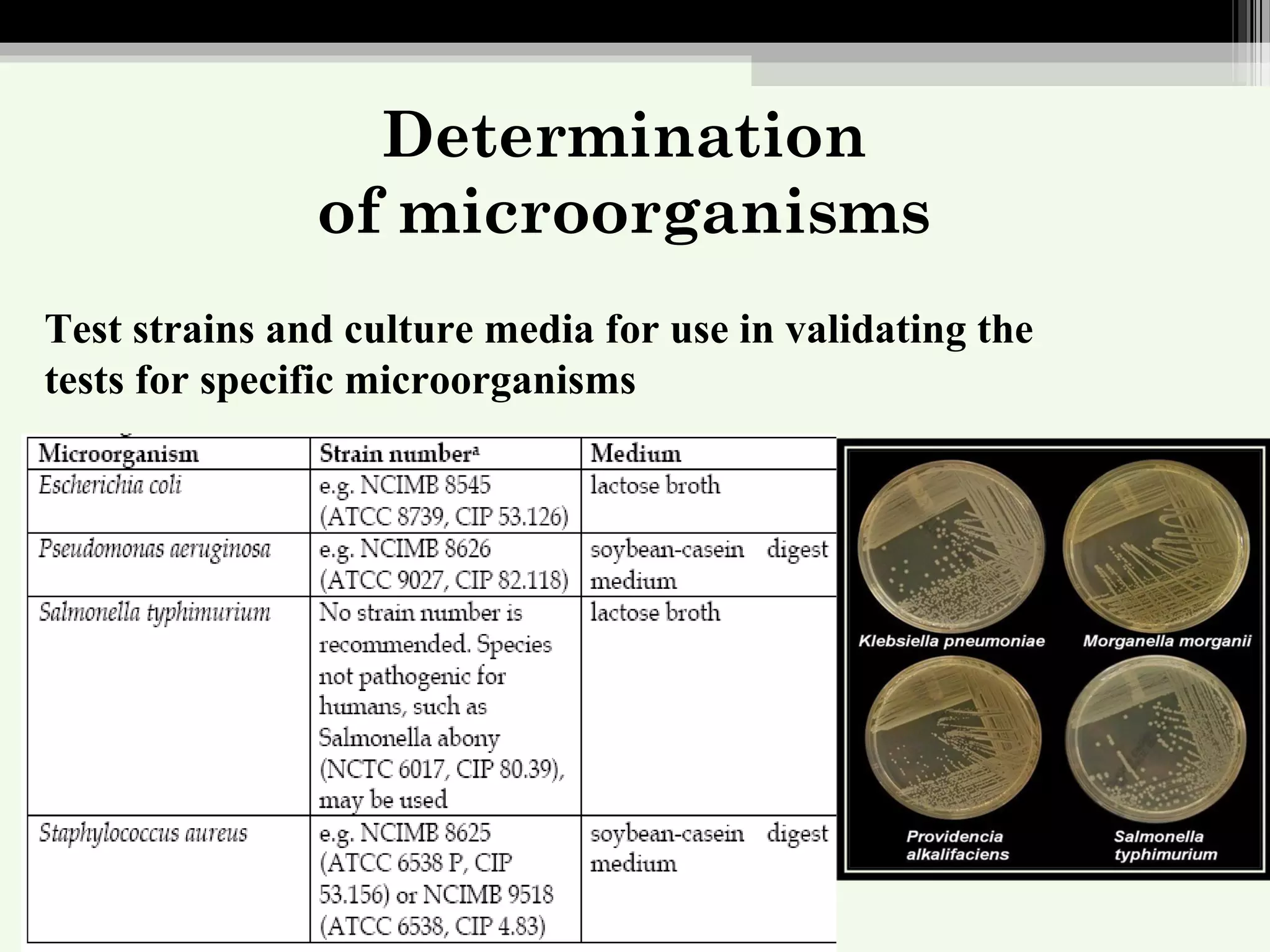
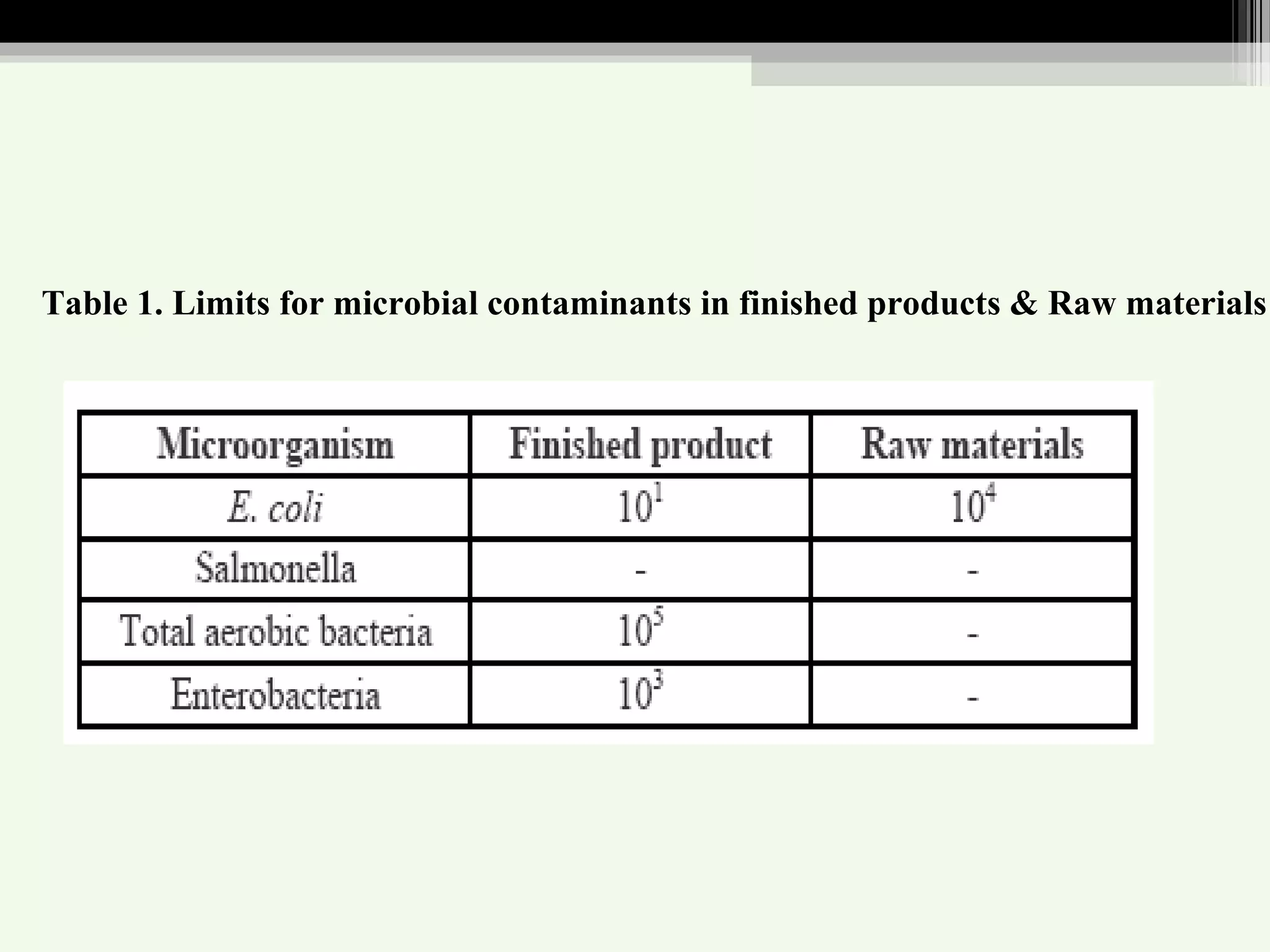
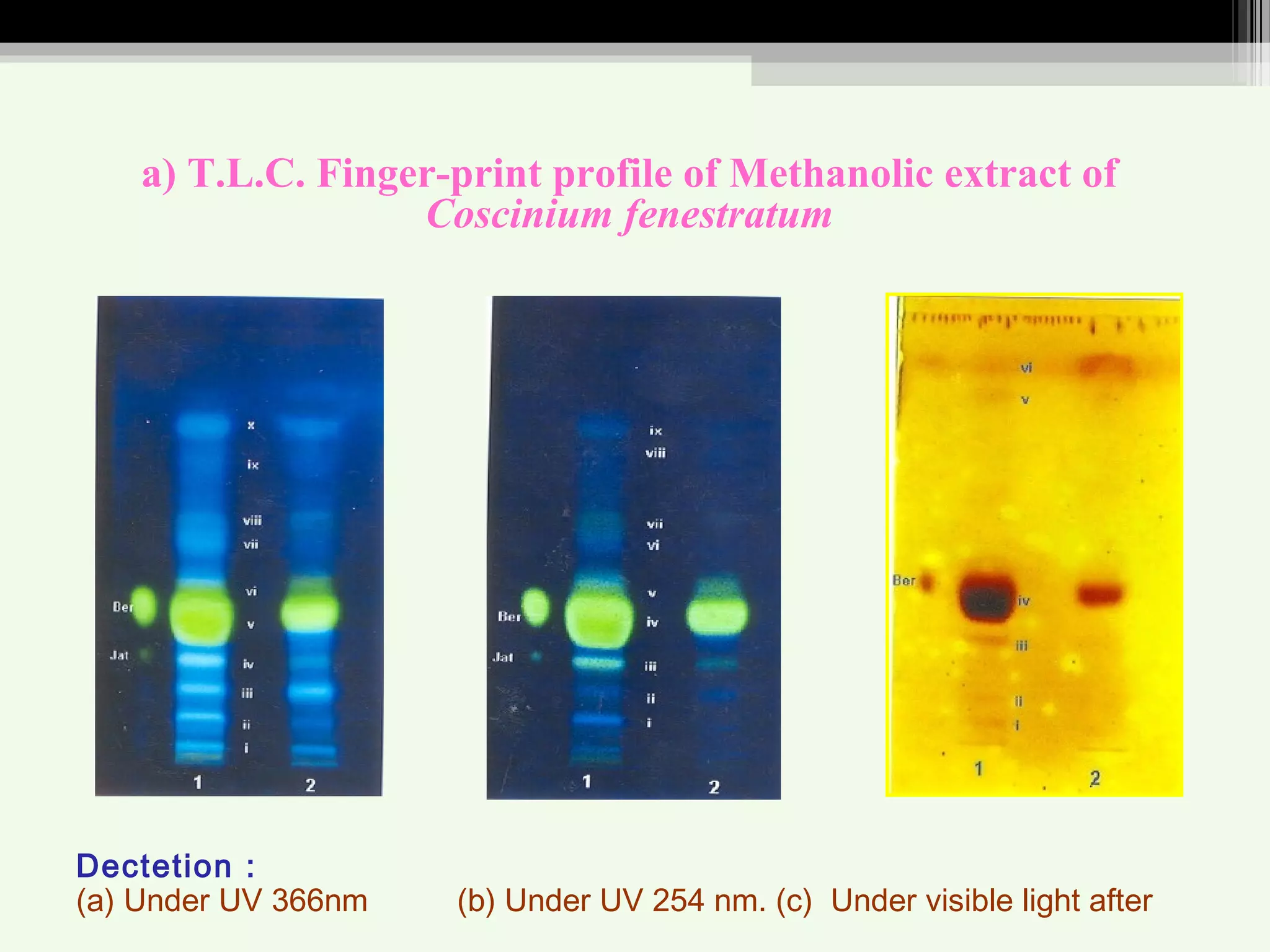
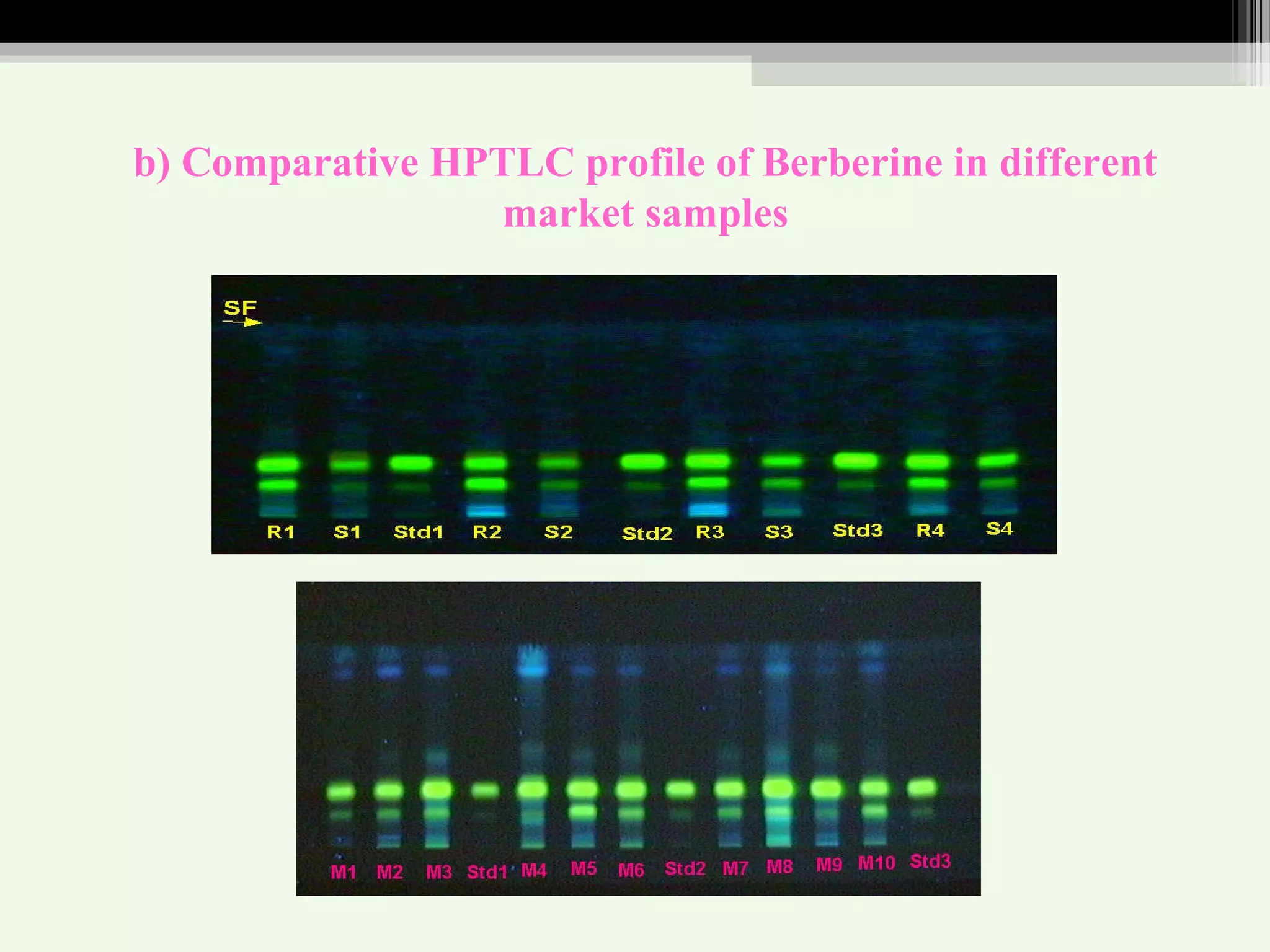
The document discusses guidelines for standardization and quality control of herbal drugs and formulations. It describes various parameters for standardization including macroscopic, microscopic, physical, chemical and biological evaluations. Specific tests covered include determination of foreign matter, ash values, extractive values, water soluble ash, total solid content, water content, volatile oil content, bitterness value, haemolytic activity, tannin content, swelling index and foaming index. Standardization helps in confirmation of identity, quality and purity of herbal drugs.