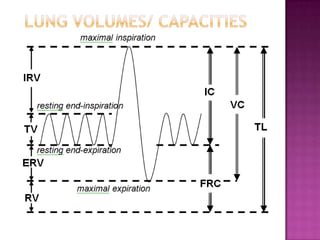
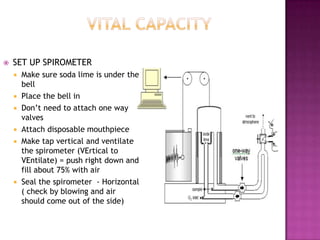
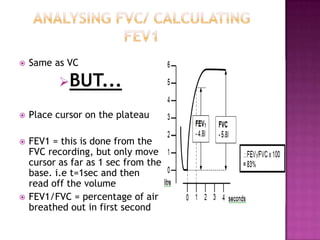
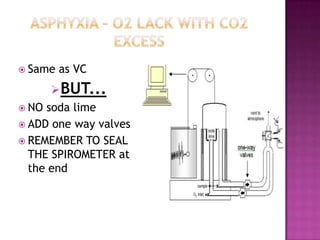
This document provides instructions on setting up and using a spirometer and PowerLab software to measure lung volumes and capacities, including vital capacity, forced vital capacity, and experiments involving asphyxia, hypercarbia, and hypoxia. It details how to configure the spirometer and software, perform tests and measurements, and analyze results. Tests include vital capacity, forced vital capacity, experiments with and without soda lime, varying the gas used to fill the spirometer, and using valves. Measurements include volumes, flows, frequencies, and gas concentrations from samples.