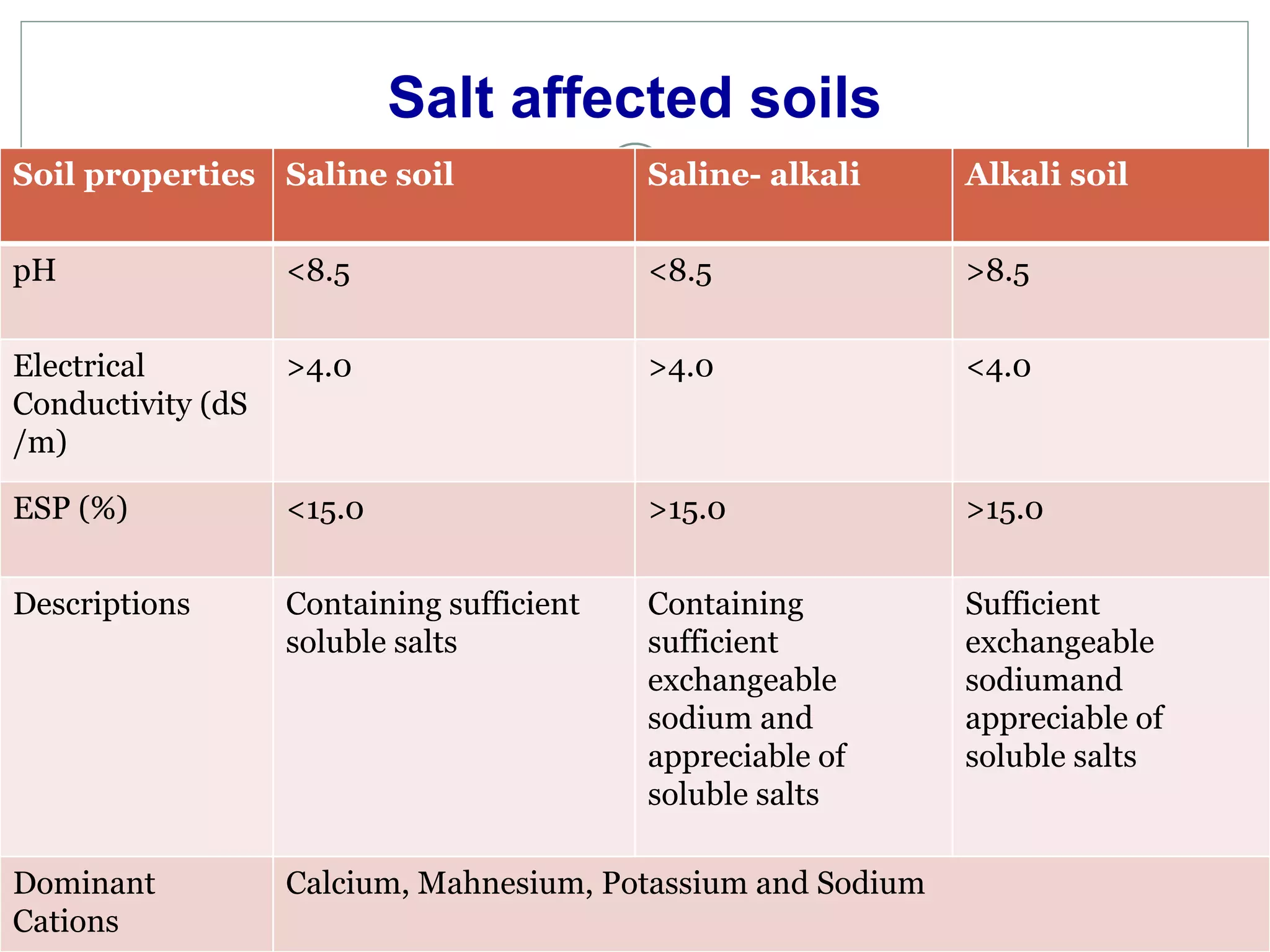
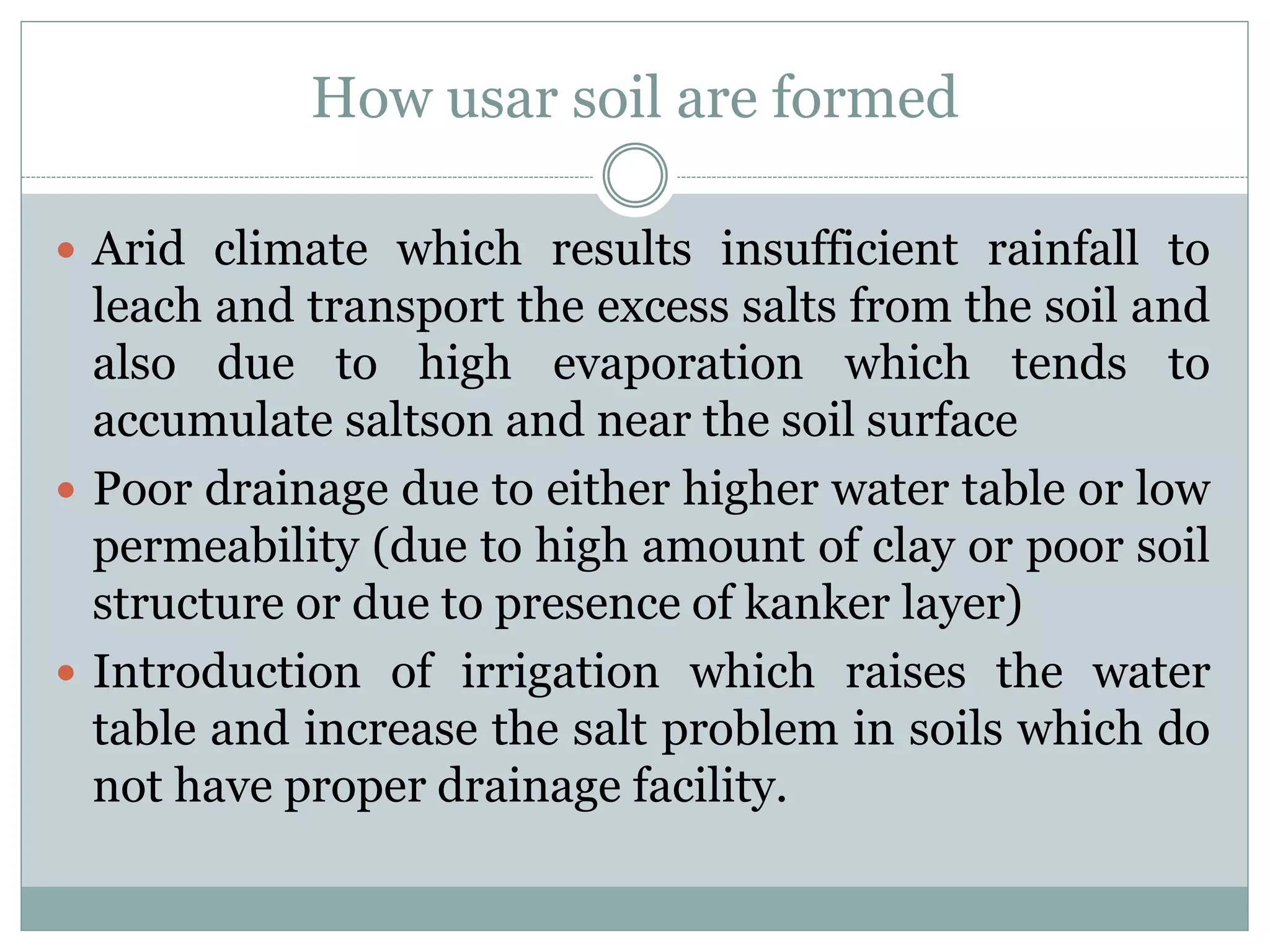
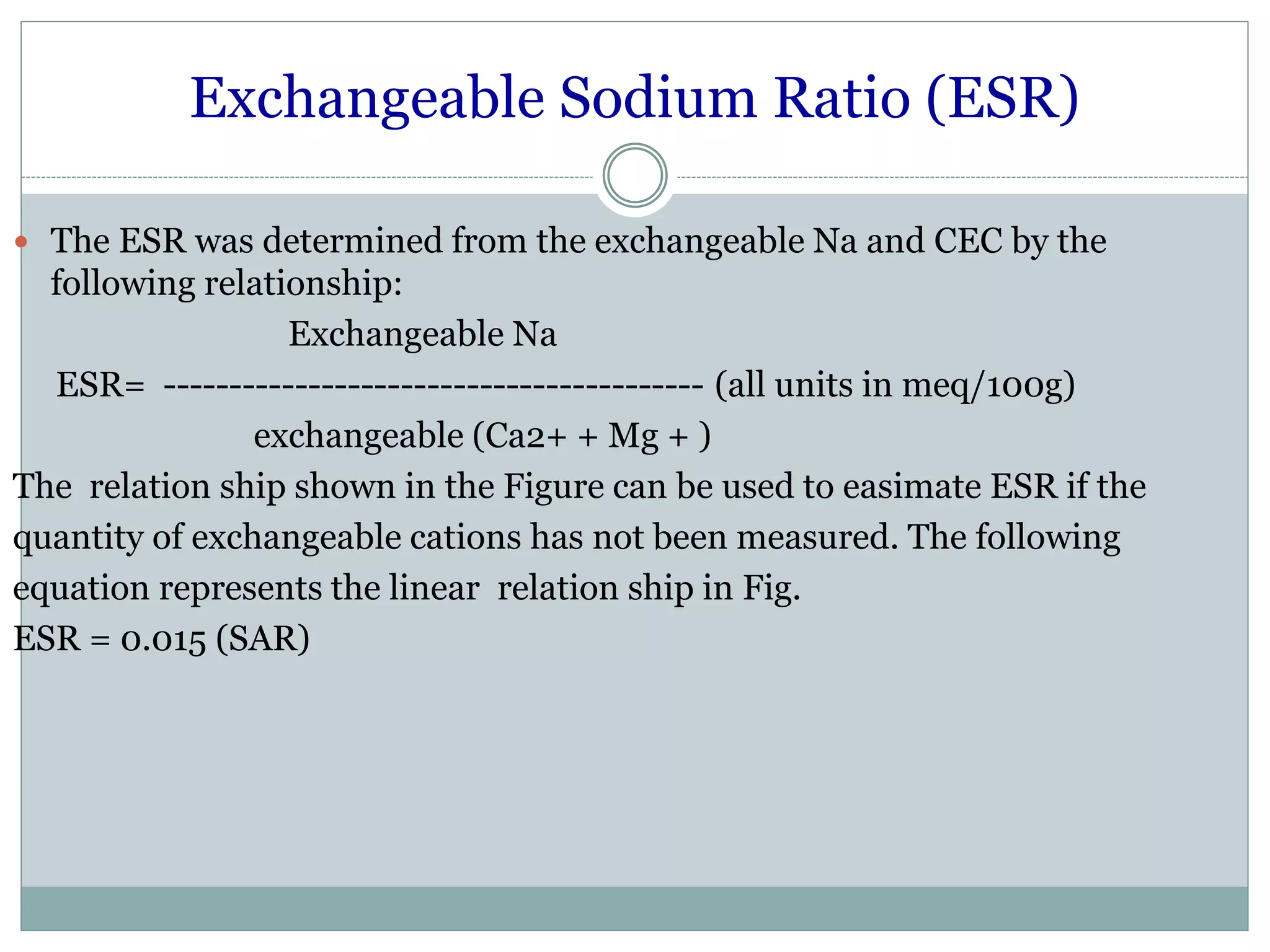
This document discusses salt-affected soils, including their classification, distribution in India, and properties. It describes saline soils, saline-alkali soils, and alkali soils based on pH, electrical conductivity, and exchangeable sodium percentage. The major causes of salt-affected soils are arid climate, poor drainage, irrigation with saline water, and other factors. Reclamation methods include physical, biological, and chemical approaches like using gypsum. Proper management of these soils requires attention to irrigation, drainage, amendments, and crop choices.