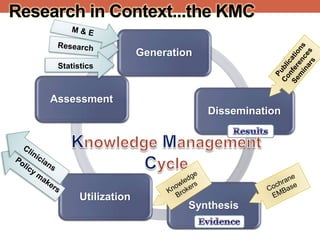
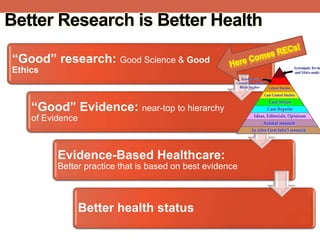
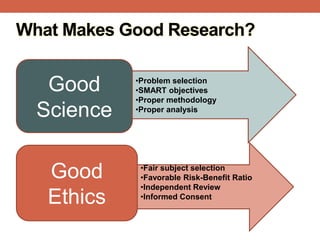
The document provides an overview of research ethics, including historical milestones, definitions, and criteria for ethical research involving human subjects. It discusses the importance of good science and ethics, outlining core principles, ethical issues, and various forms of research misconduct such as fabrication and plagiarism. Additionally, it offers guidance on how to maintain ethical standards and avoid misconduct throughout the research process.


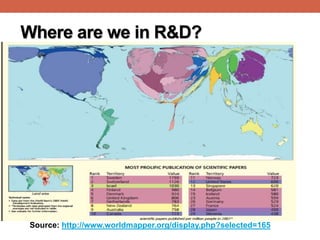
![Where are we in R&D?
Expenditure on R&D as % of
GDP (2013):[1]
• Arab world: 0.5%
• China (2%),
• EU (2.3%),
• USA (2.8%),
• Israel (4%)
Number of researchers (per
1,000,000 population) [2]
• Morocco : 864
• Argentina: 1,236
• Malaysia: 1,643
• Slovenia: 4,255
• Israel: 6,494
Published scientific papers (1996 -2013):[3]
Egypt (42nd): 104,784 Brazil: 529,841
Israel: 247,561 India: 868,719
Turkey: 348,836 USA: 7,846,972](https://image.slidesharecdn.com/researchethicsscientificmisconduct-fmoh04-150310093542-conversion-gate01/85/Research-ethics-scientific-misconduct-4-320.jpg)





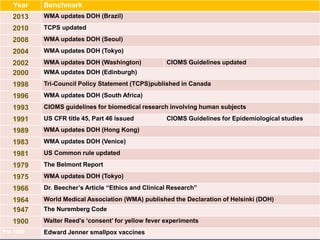
![History of Research Ethics
Pre-World War II: Research standards left up to the discretion
of the individual researcher
18th and 19th Centuries
• James Lind “scurvy study in sailors - Salisbury
• Edward Jenner cowpox vaccine test
• 1897 Giuseppe Sanarelli yellow fever test
1900 Walter Reed established several [first ever]
“safeguards”
• Self-experimentation
• Only adults would be enrolled in research
• Written informed consent
• Reimbursement (inducement)](https://image.slidesharecdn.com/researchethicsscientificmisconduct-fmoh04-150310093542-conversion-gate01/85/Research-ethics-scientific-misconduct-13-320.jpg)