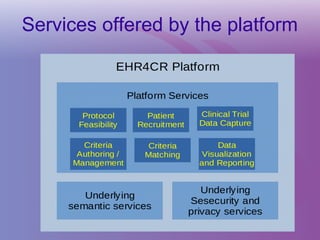
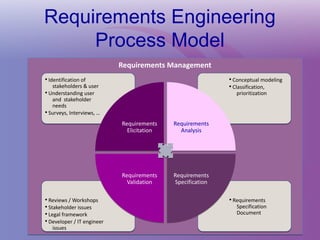
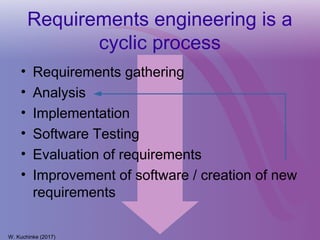
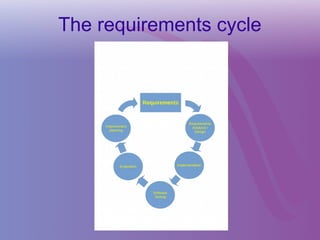
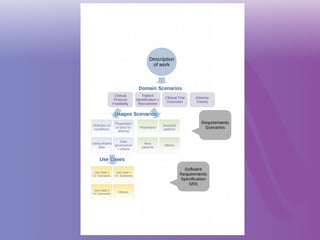
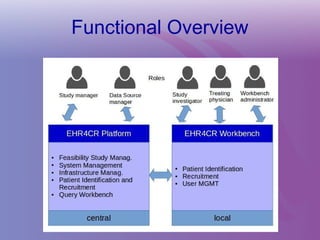
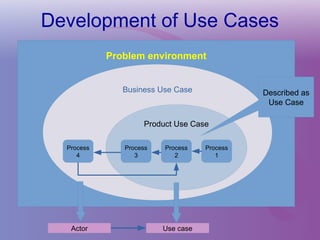
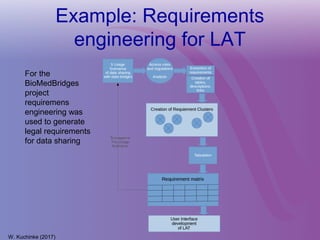
The document discusses a workshop on requirements engineering, focusing on the successful methodologies used in the ehr4cr project aimed at promoting the reuse of electronic health records for clinical trials across Europe. It outlines the iterative processes of requirements gathering, analysis, specification, and validation, emphasizing the importance of stakeholder engagement and the use of scenarios for effective requirements management. The workshop also highlights the development and application of tools, platforms, and strategies necessary for enhancing clinical research through improved requirements engineering practices.