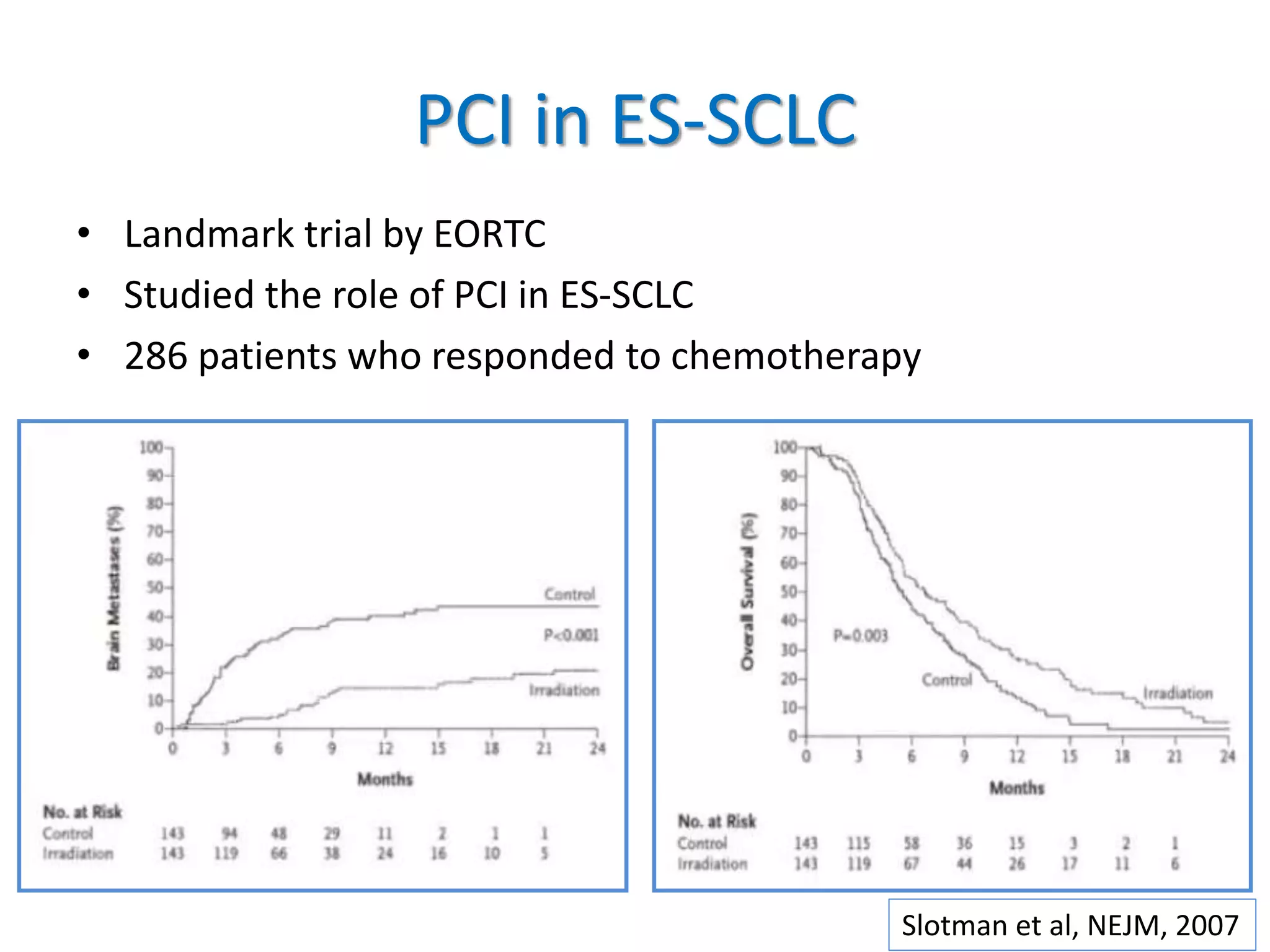
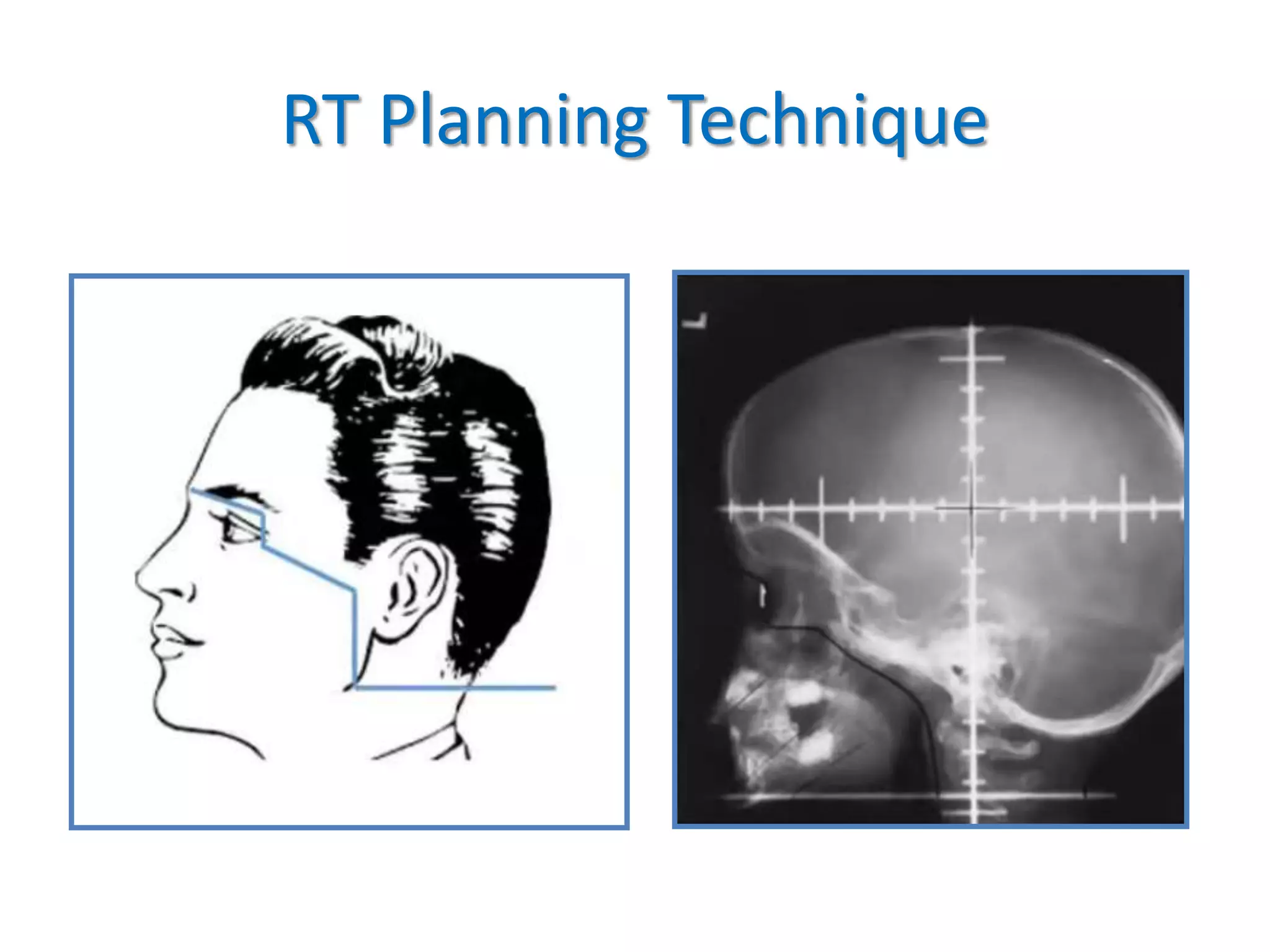
Prophylactic cranial irradiation (PCI) is used to prevent brain metastases in cancers with a high risk of spreading to the brain. It is indicated for small cell lung cancer and certain leukemias. PCI significantly reduces the rate of brain metastases in small cell lung cancer, especially when administered early at higher doses. For extensive stage small cell lung cancer, MRI surveillance may be an alternative to PCI. While PCI reduces brain metastases in leukemia, the risk of brain involvement is low for some types such as AML. The standard dose for PCI is 1200-1800 cGy in fractions, with timing and volumes depending on the cancer type. Potential toxicities include neurocognitive effects, endocrine disorders, and secondary cancers.