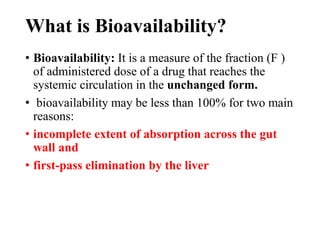
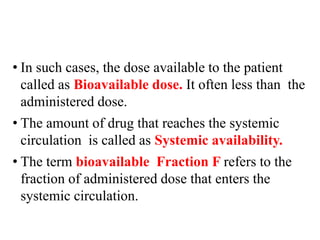
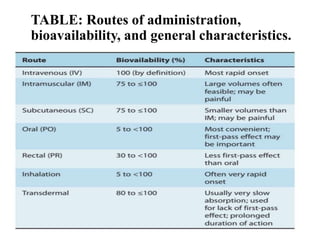
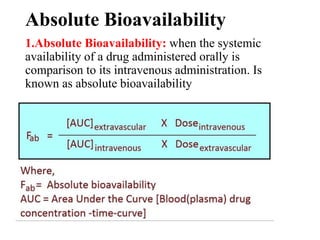
Bioavailability is a measure of the amount of drug that reaches systemic circulation after administration. It may be less than 100% due to incomplete absorption and first-pass metabolism by the liver. Factors like pharmaceutical properties, gastrointestinal conditions, food intake, and route of administration can impact bioavailability. There are two types - absolute bioavailability compares systemic availability after oral vs intravenous doses, while relative bioavailability compares oral doses to an oral standard. Bioavailability is assessed indirectly through pharmacokinetic methods like analyzing blood concentrations over time and measuring drug excreted unchanged in urine.