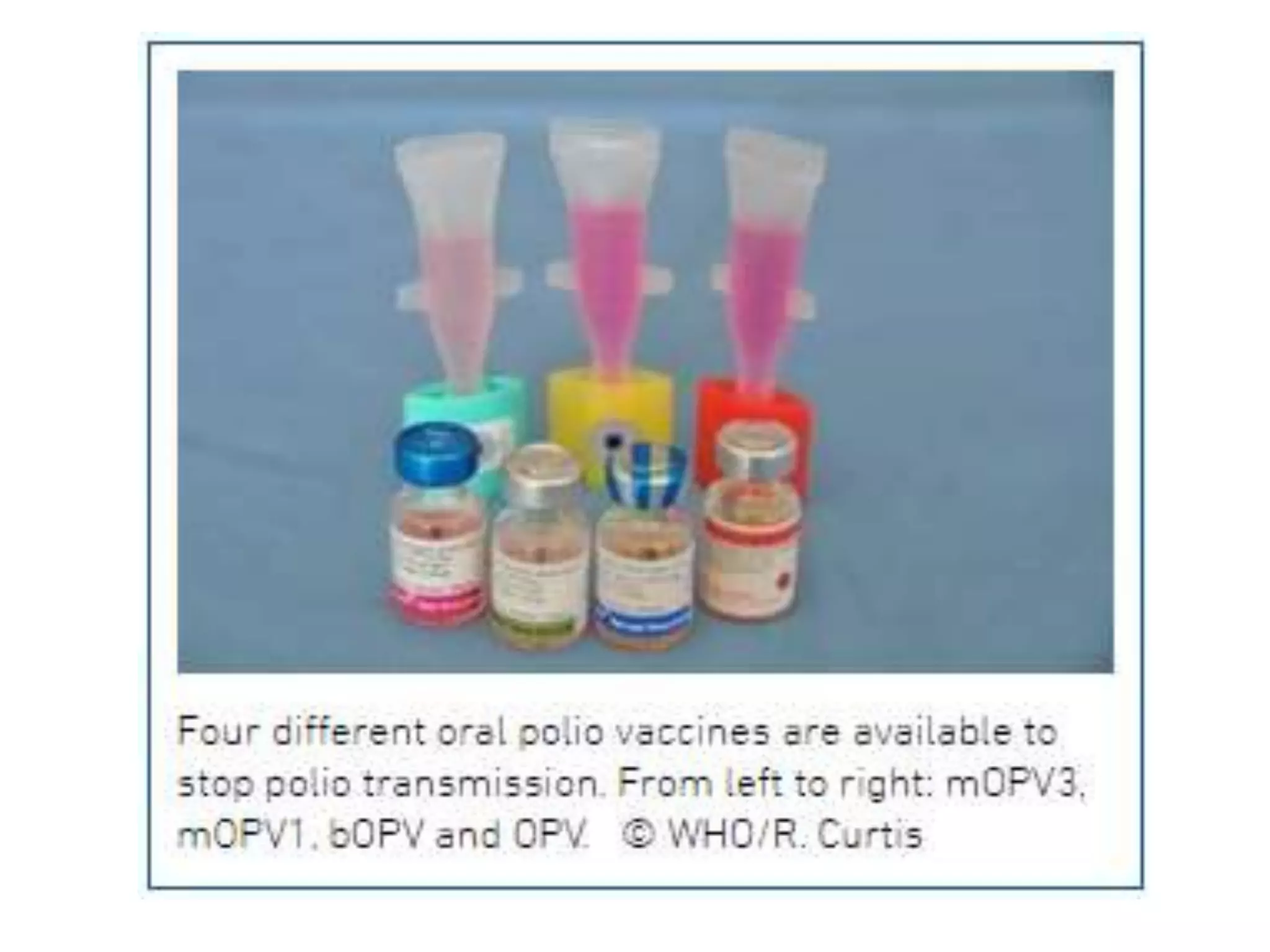
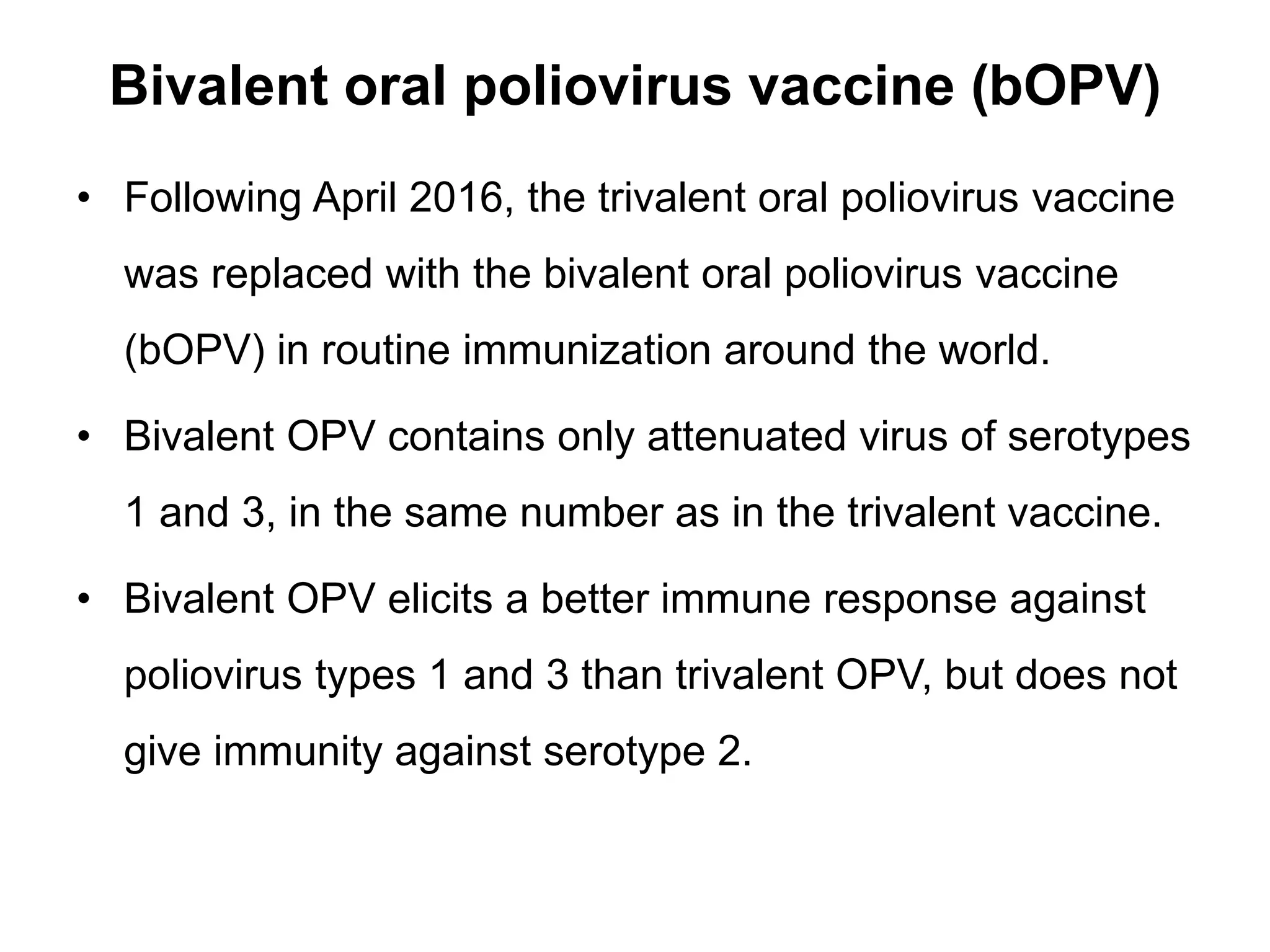
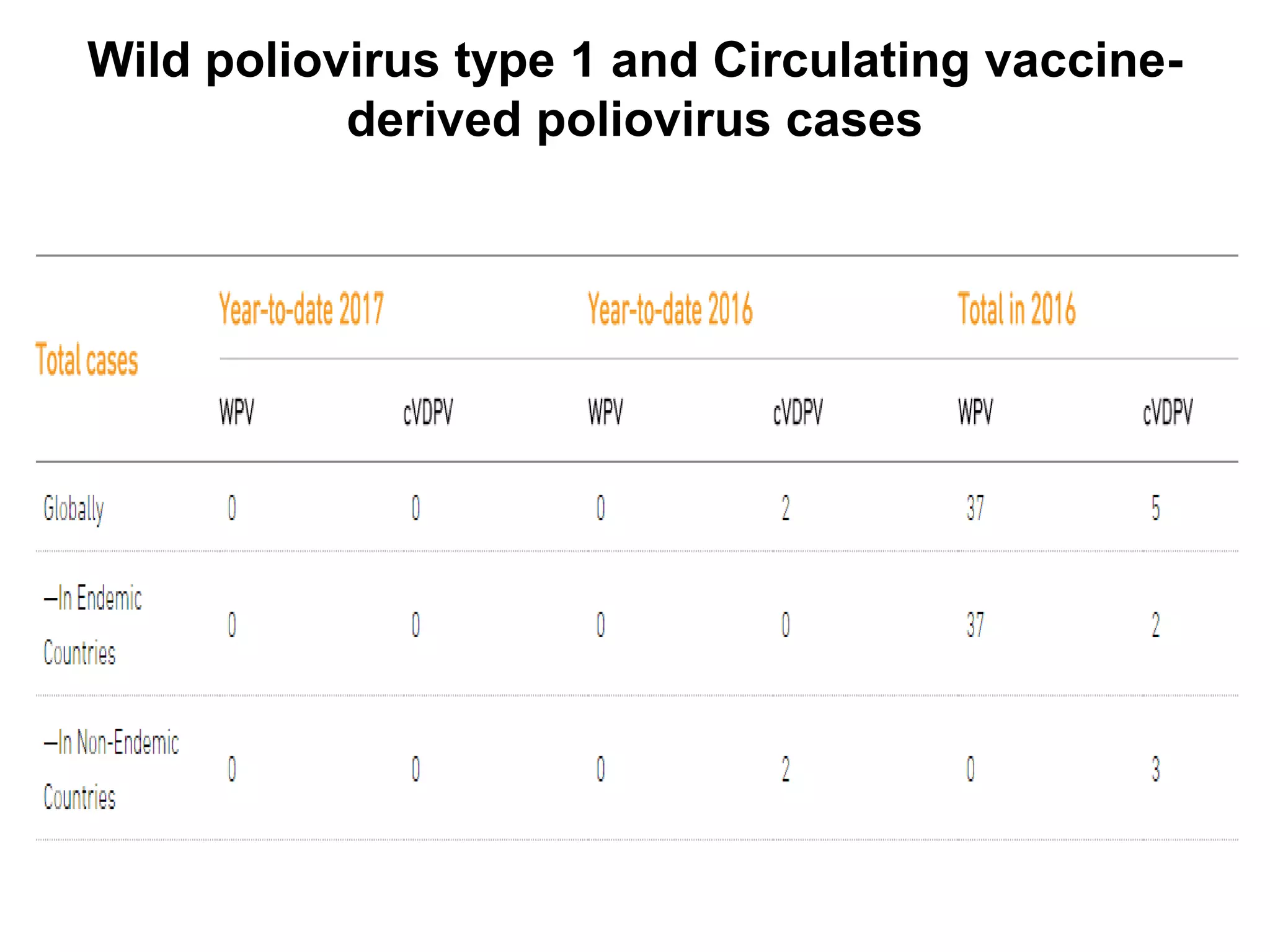
The document provides information on the current status of polio eradication efforts. While polio cases have decreased by over 99% since 1988, the disease remains endemic in Afghanistan and Pakistan, where 6 cases were reported in 2016. India was declared polio-free in 2014. Different oral and inactivated polio vaccines have been developed and used in eradication campaigns. Maintaining high vaccination rates is critical to preventing wild poliovirus and vaccine-derived poliovirus outbreaks. Surveillance indicators like acute flaccid paralysis reporting are used to detect polio cases and monitor progress toward global certification of eradication.