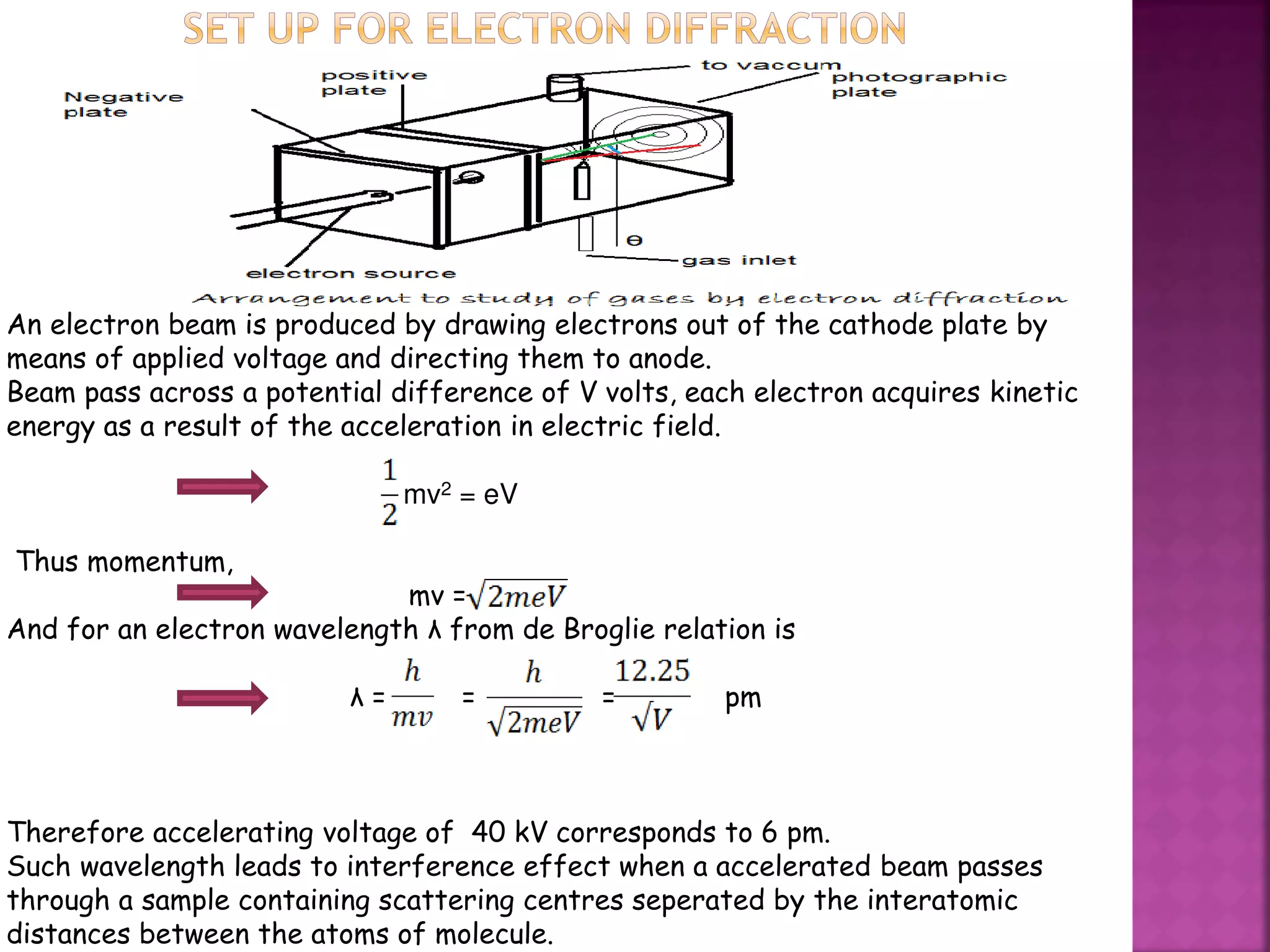
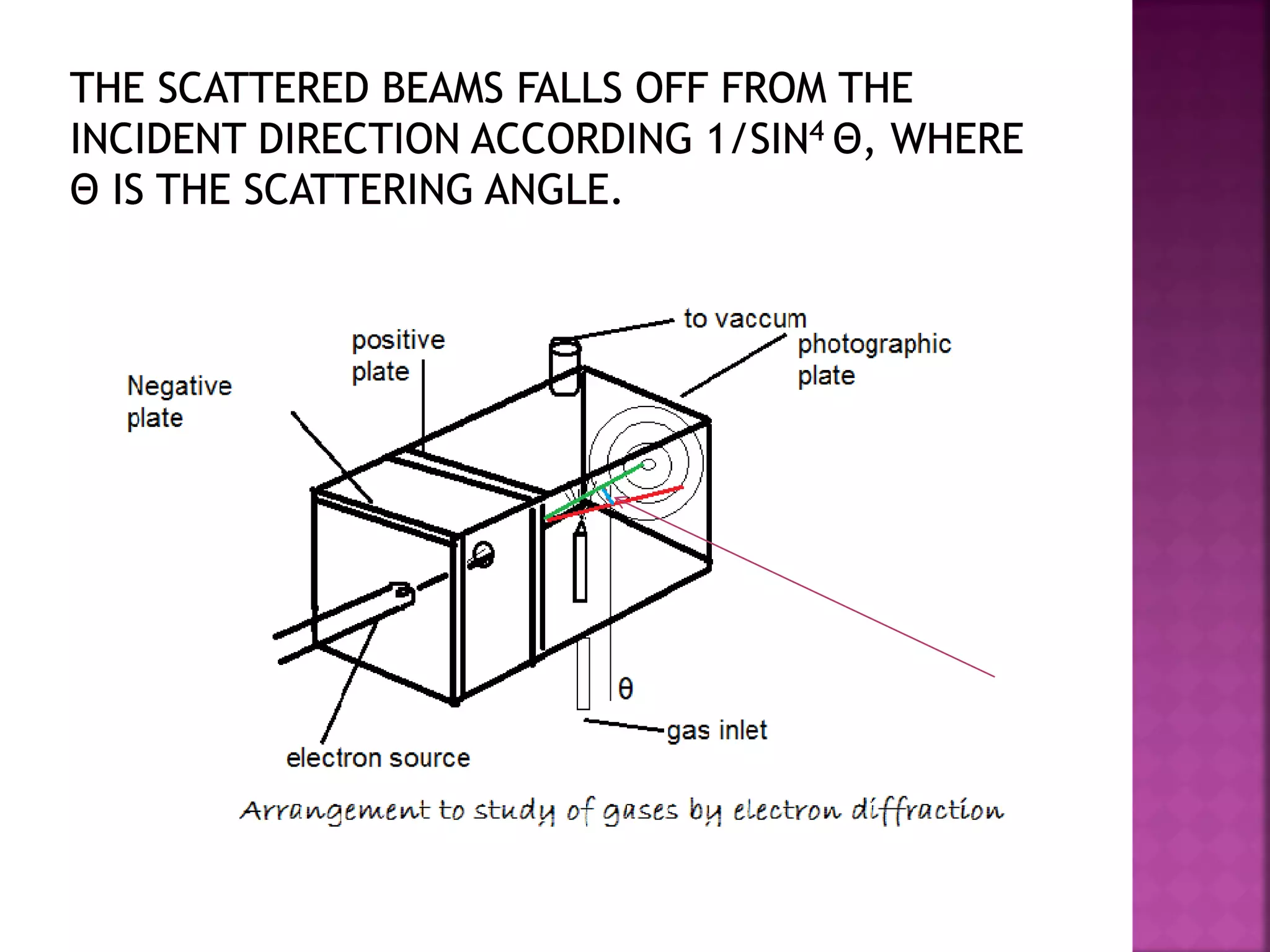
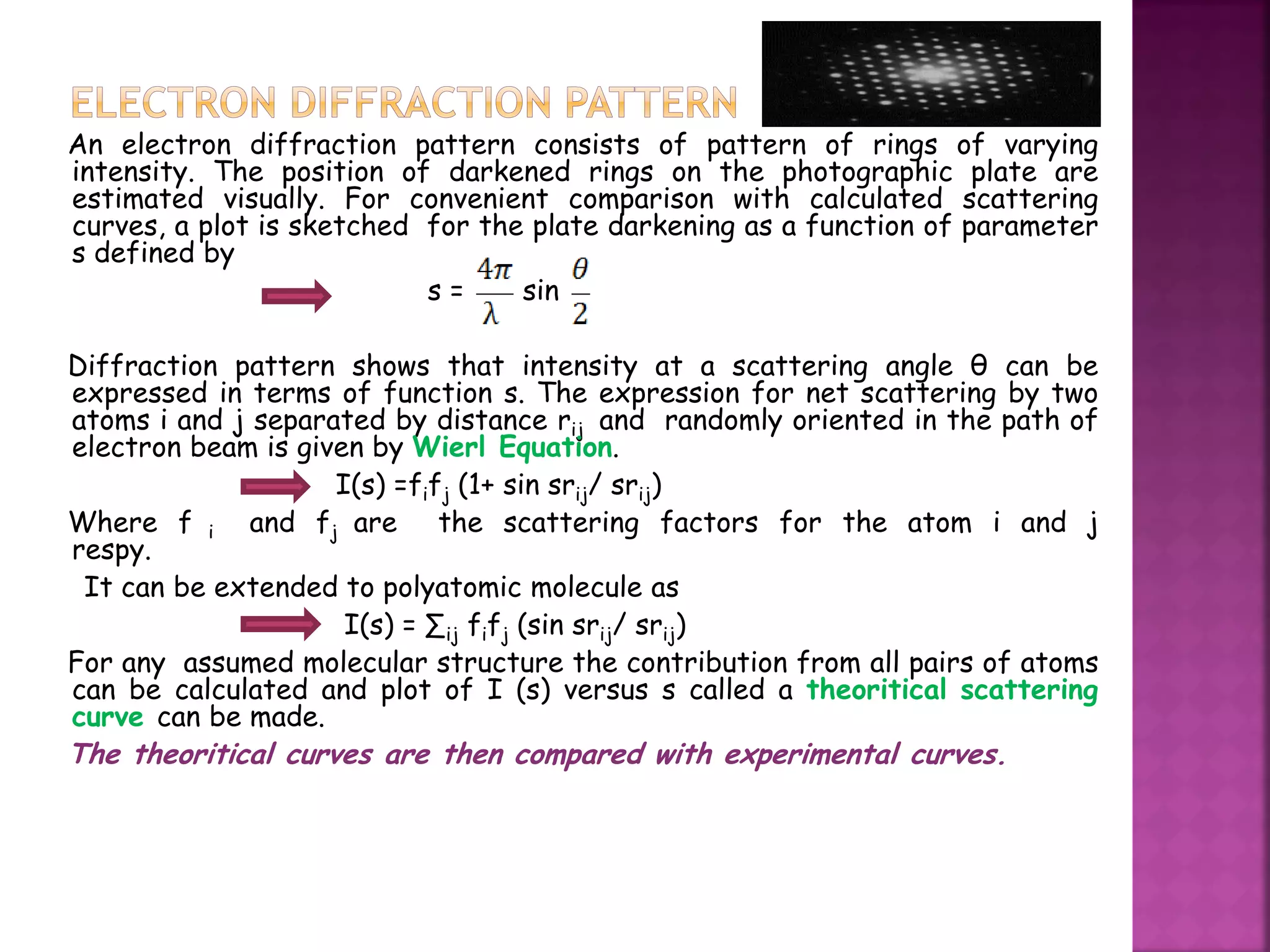
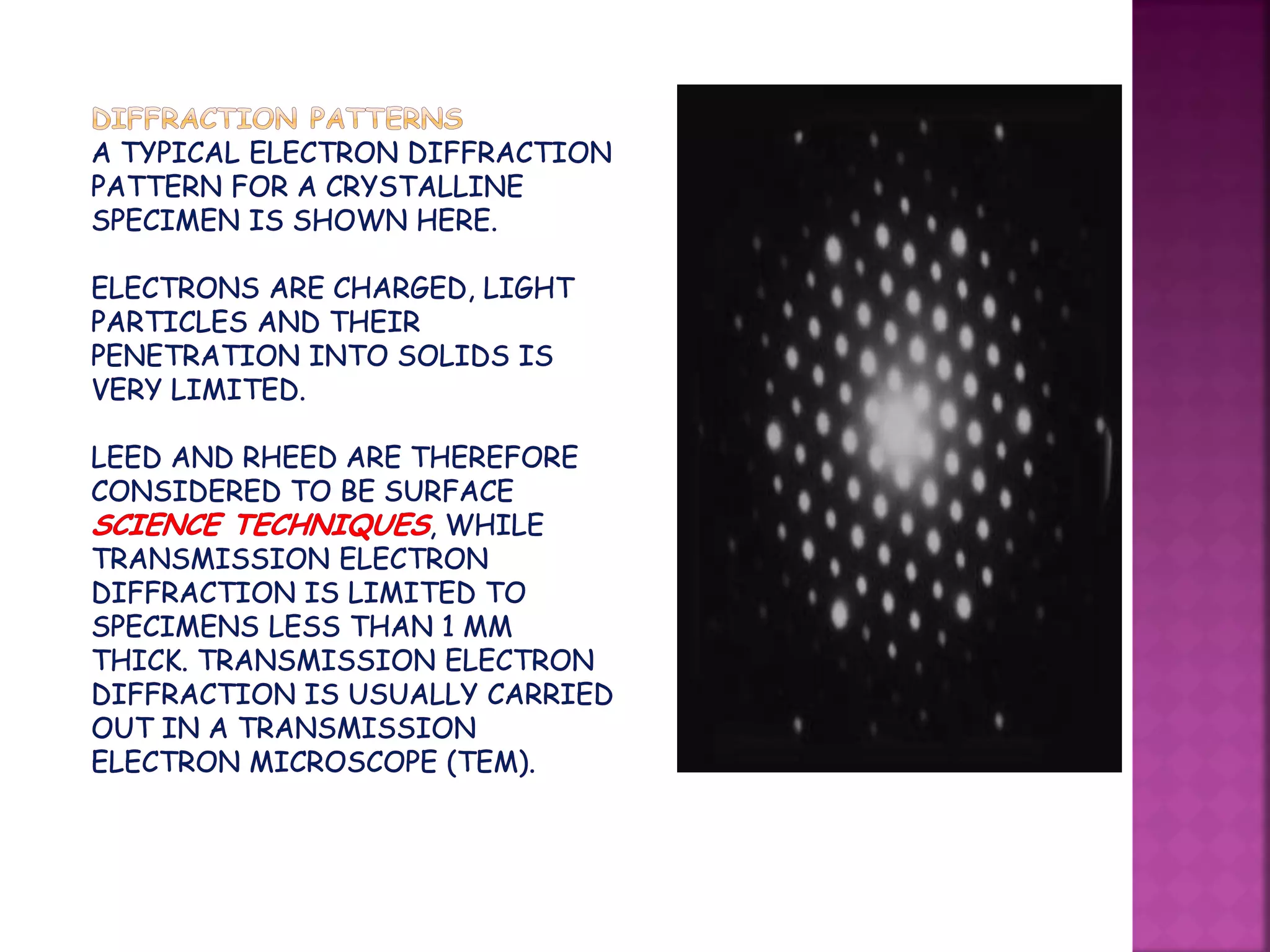
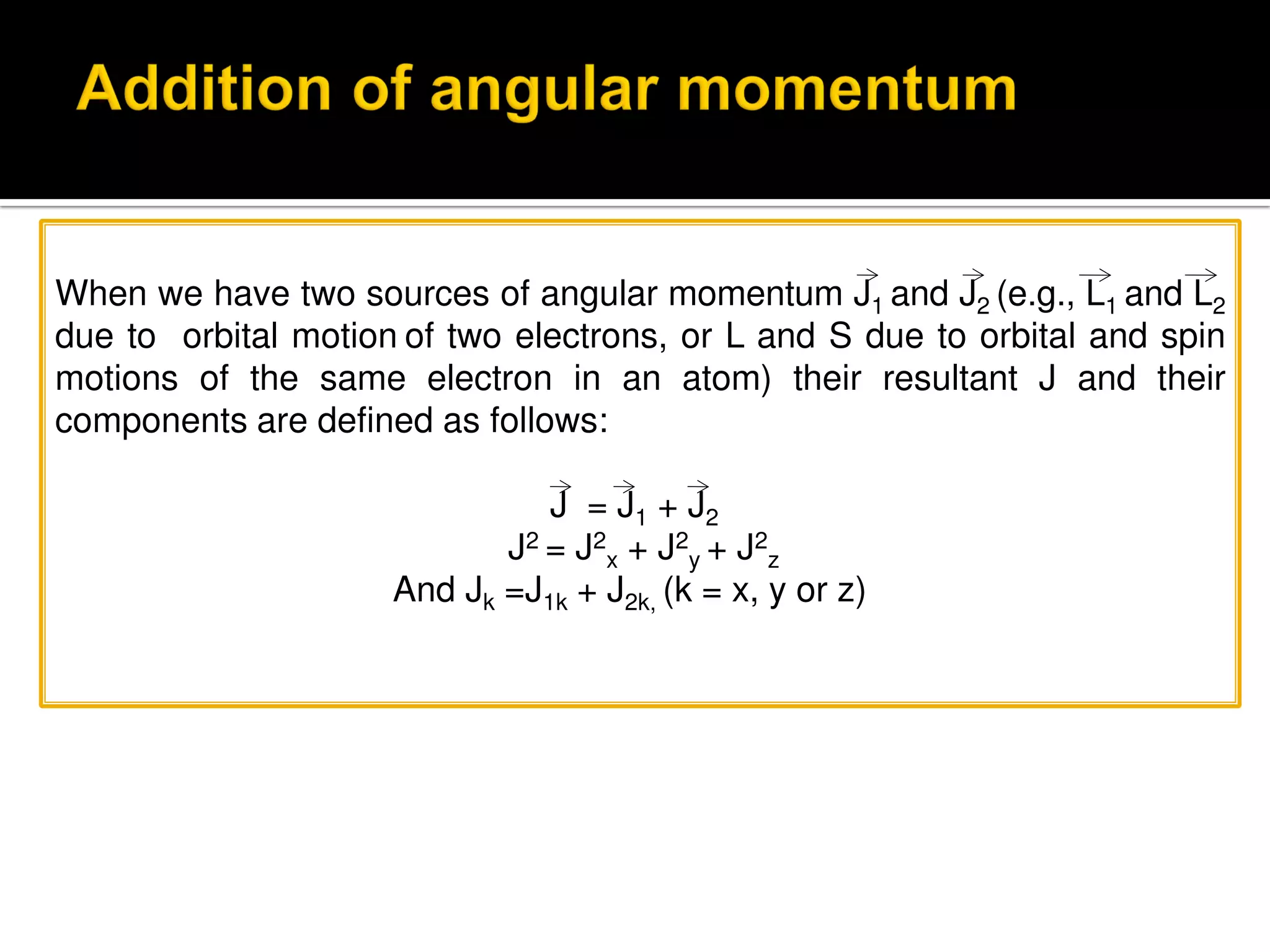
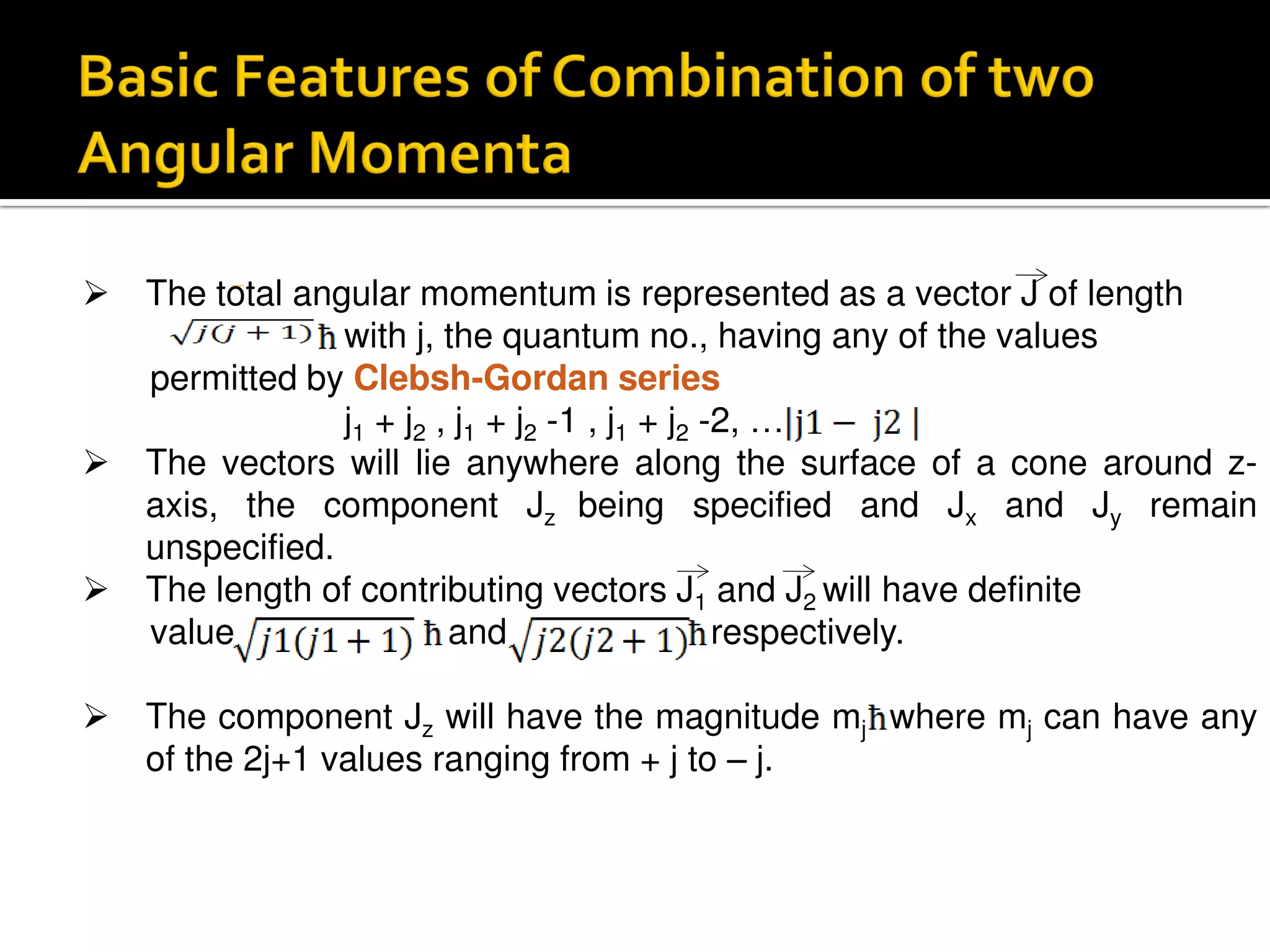
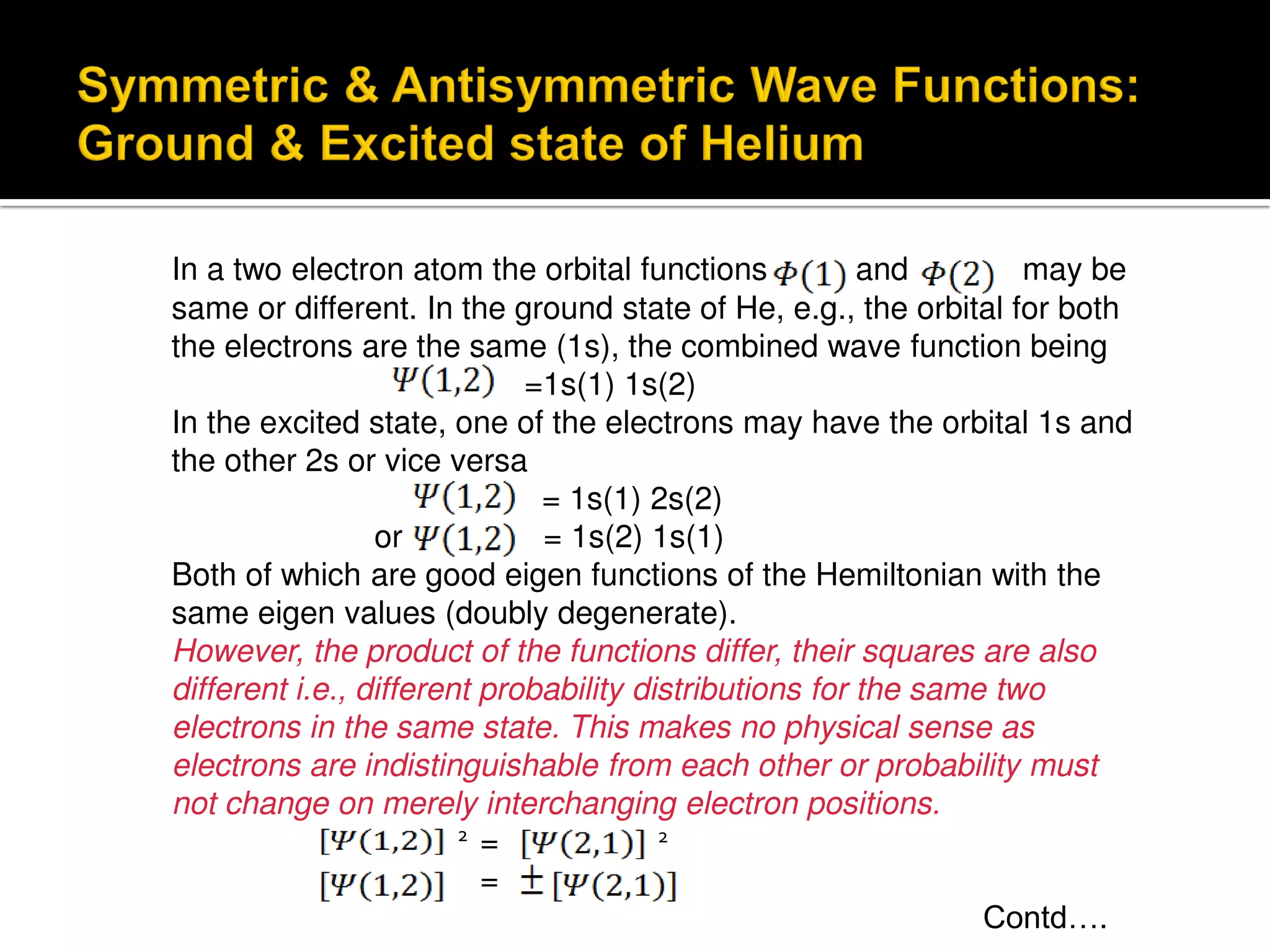
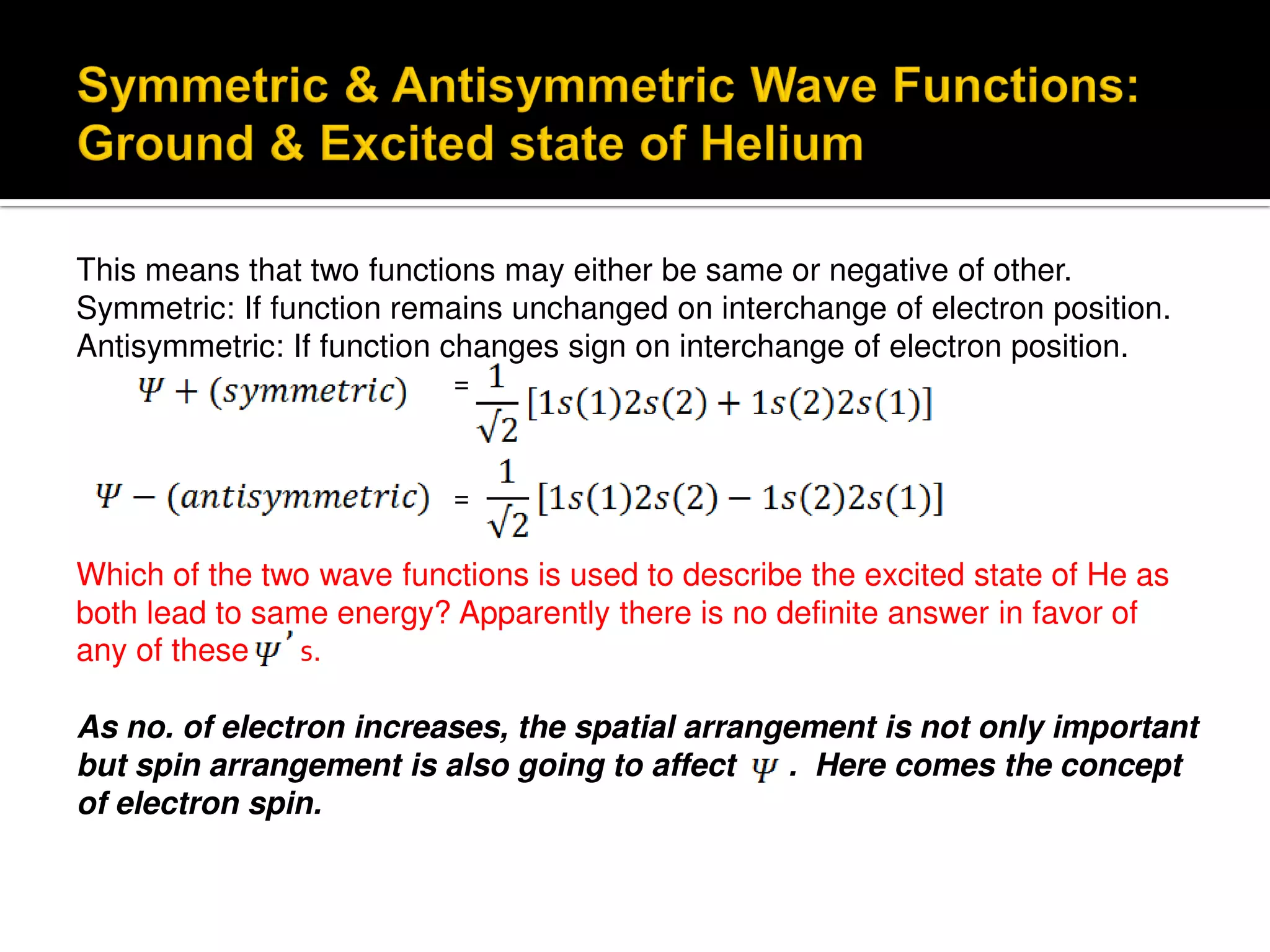
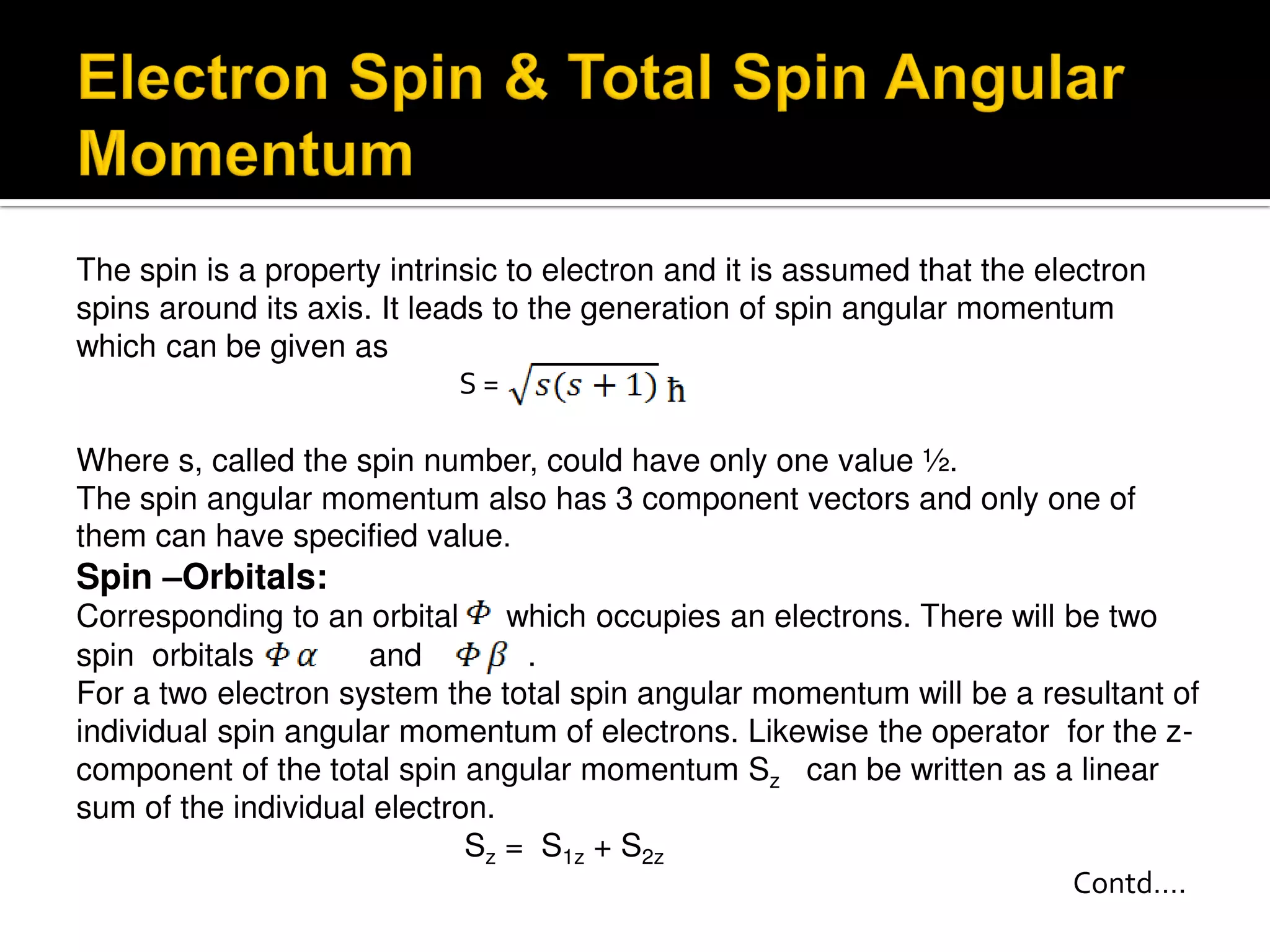
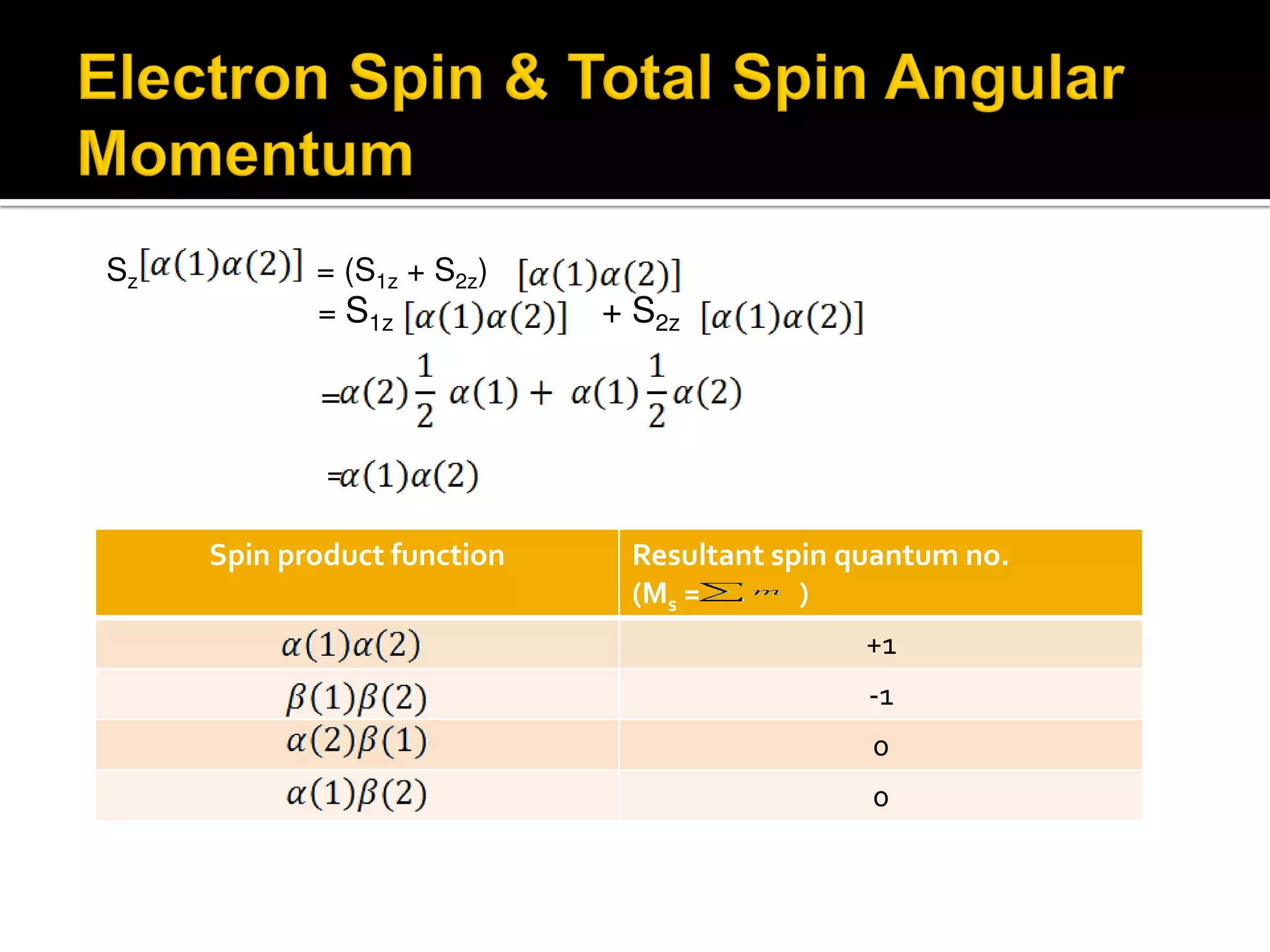
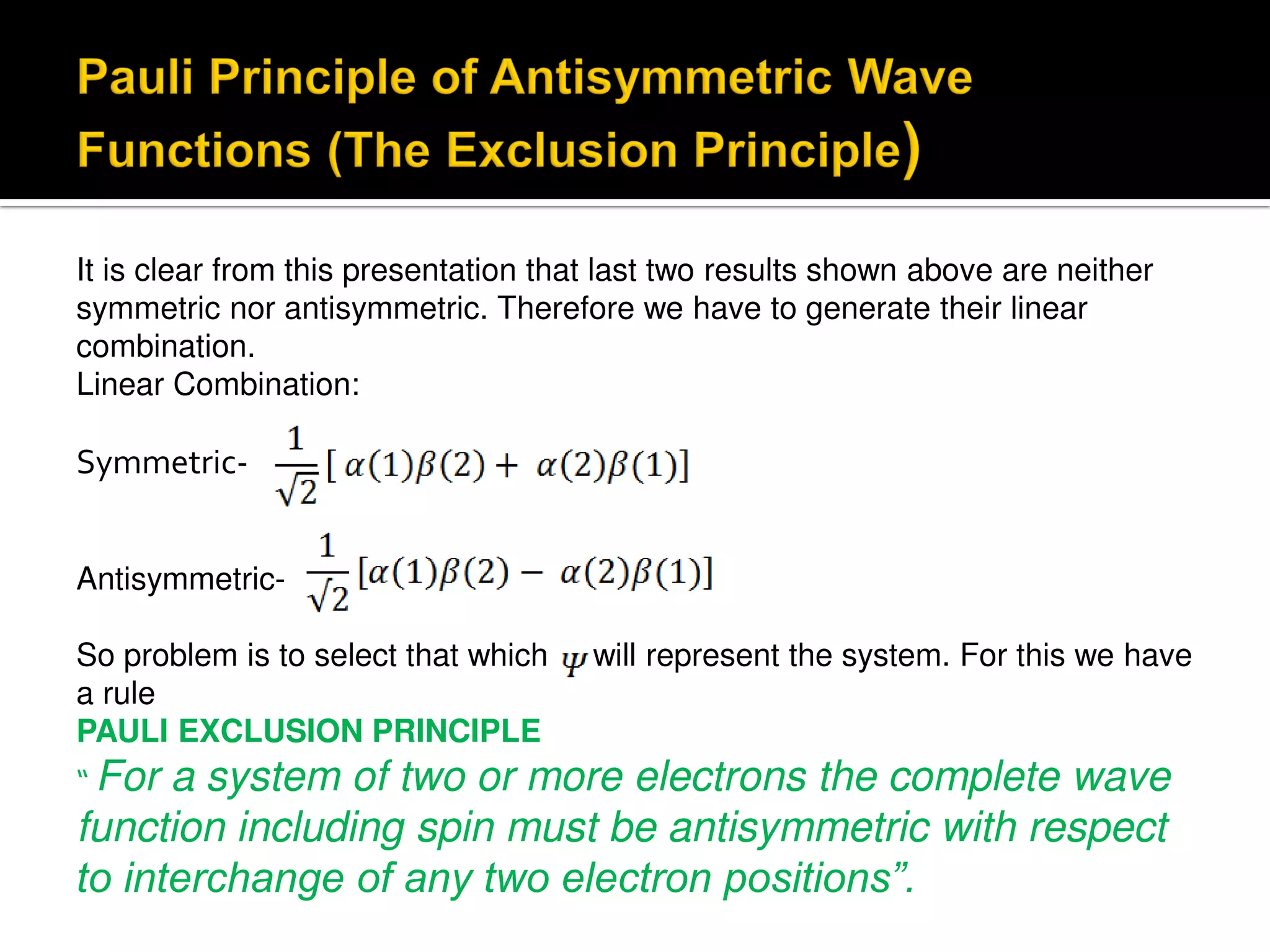
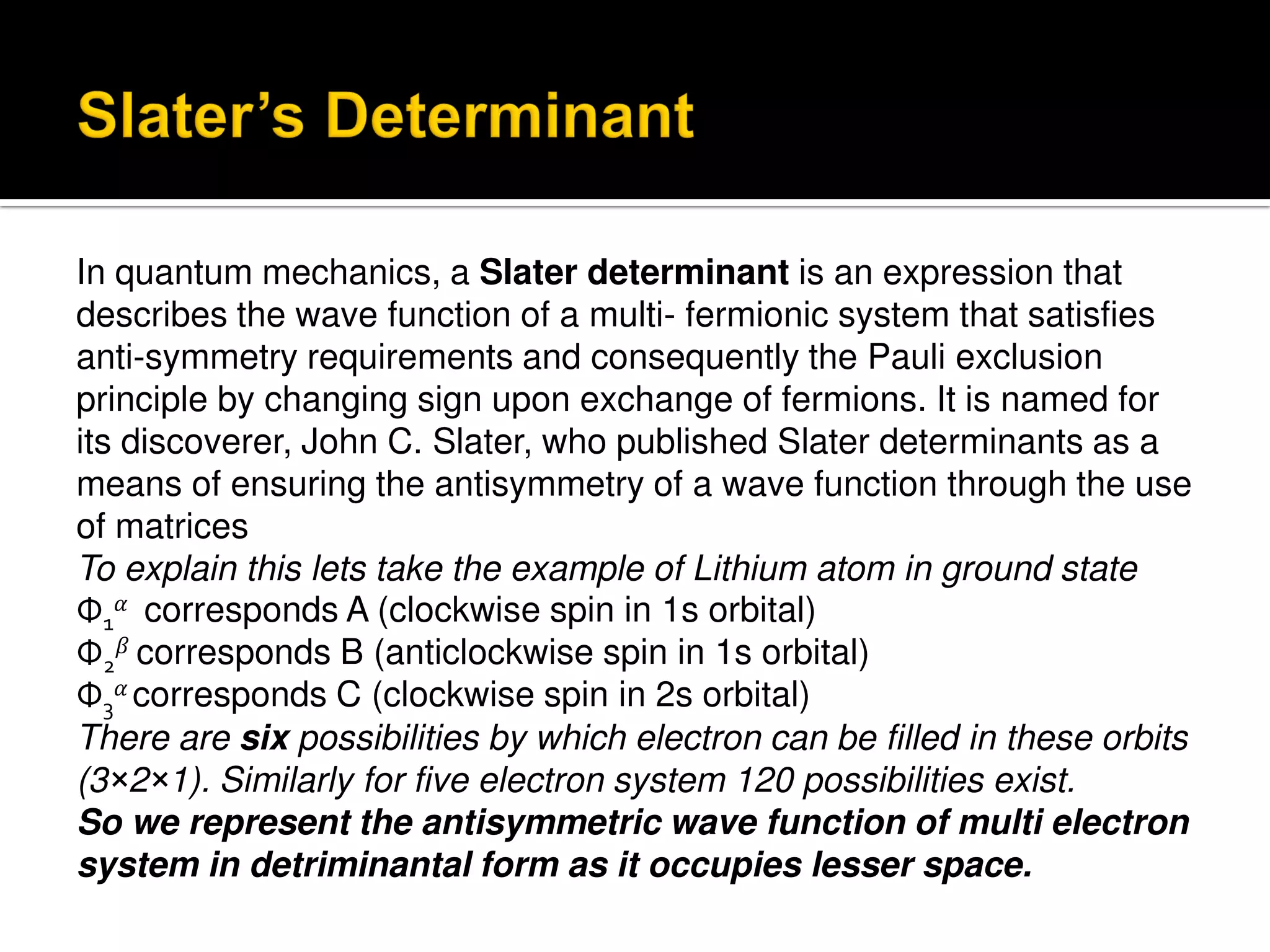
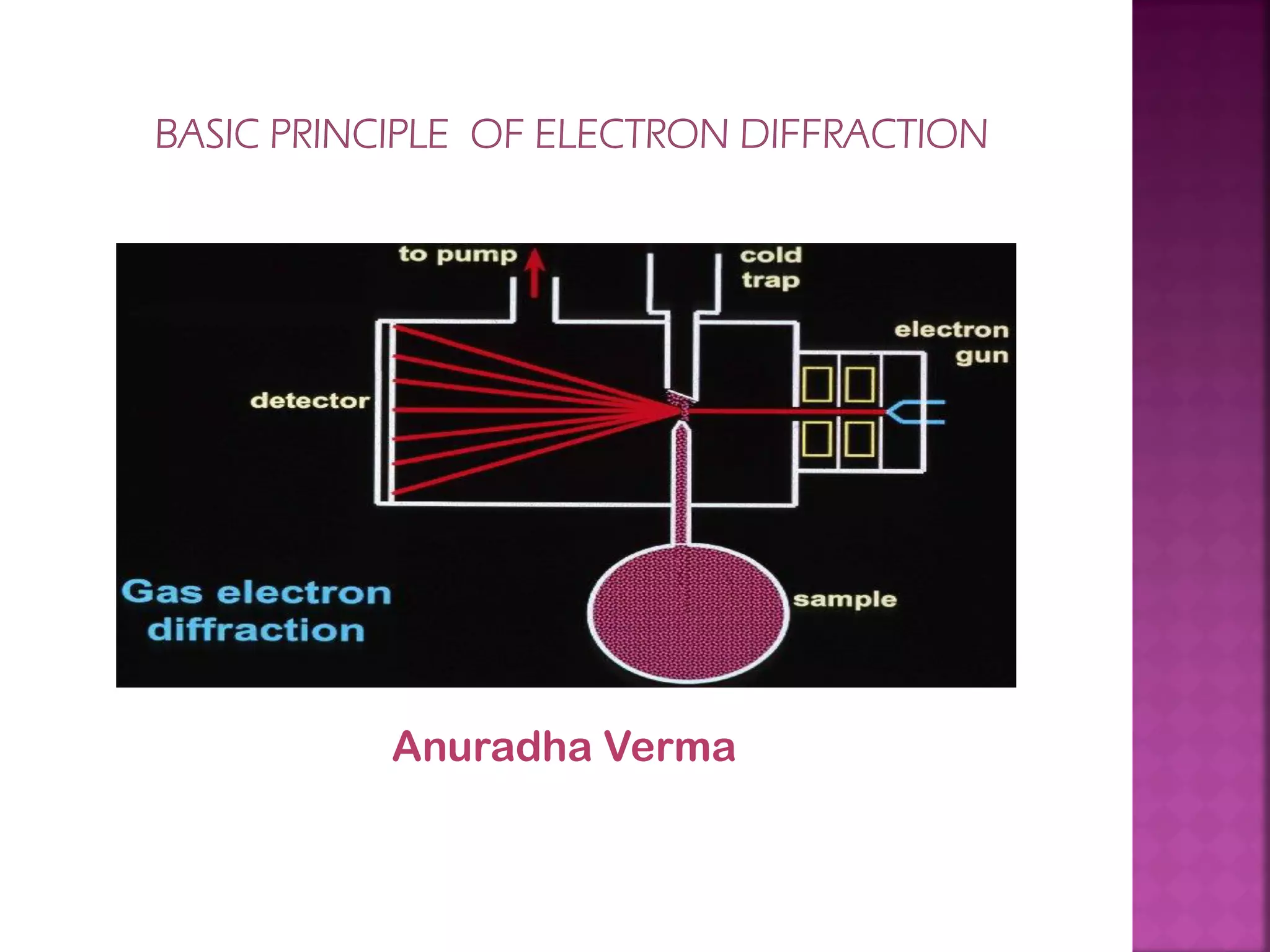
Electron diffraction is a technique that uses the wave-like properties of electrons to determine the structure of matter. When electrons are fired at a sample, they diffract according to the positions of atoms, producing an interference pattern. This pattern provides information about distances between atoms in gas molecules. Electron diffraction was first demonstrated in 1927 and was later used to determine the structure of carbon tetrachloride in 1930. Electrons interact with matter through both electrostatic and magnetic forces, allowing for probing of both atomic nuclei and surrounding electrons. The intensity of diffracted beams depends on factors like the structure factor, which incorporates the scattering power and positions of atoms in the sample unit cell.







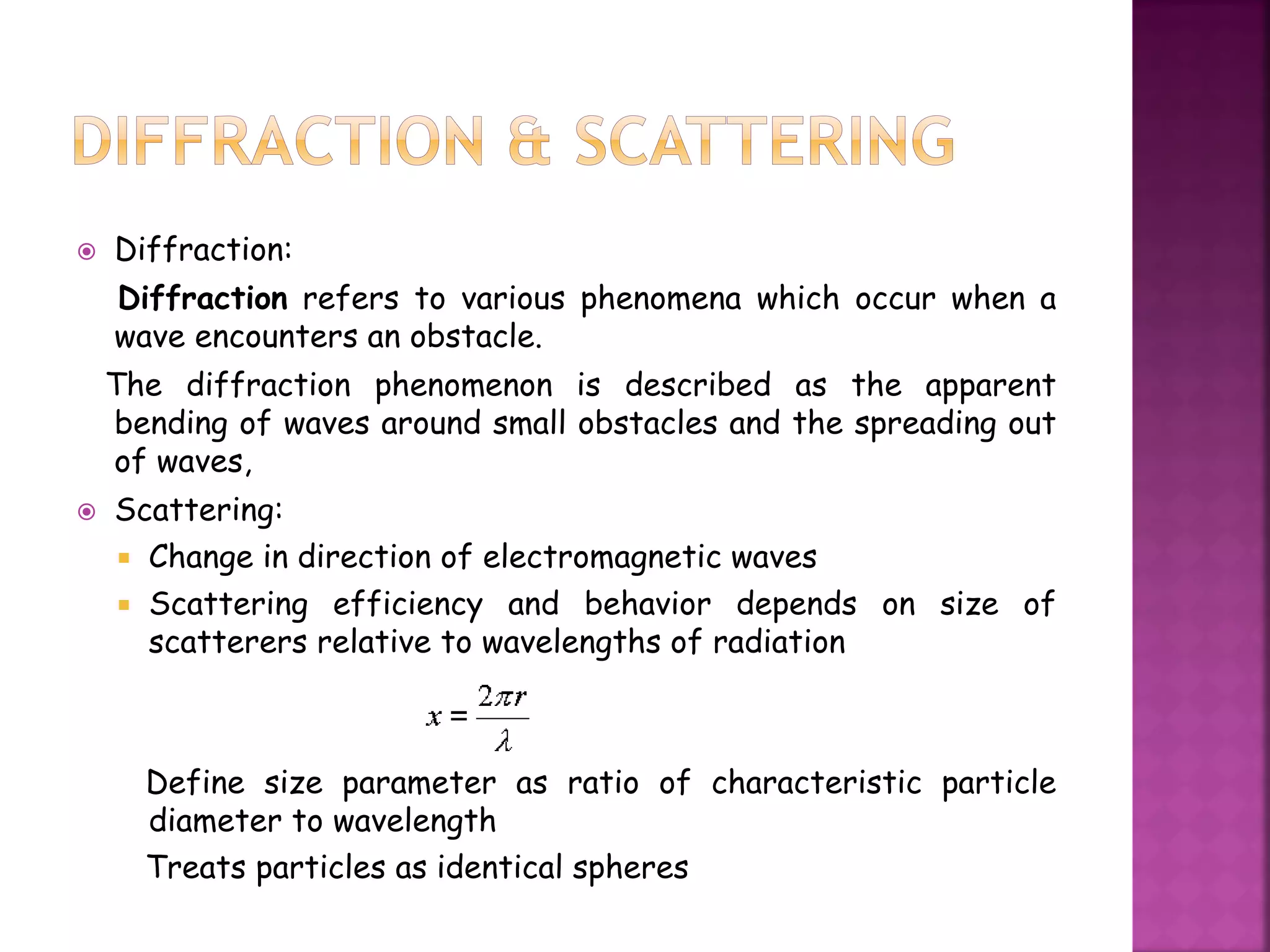
![Scattering efficiency of a particle, customarily denoted by
Qb [non-dimensional], is defined by the following equation:
Qb = Cb / G
where Cb [length2] is the scattering cross section of the
particle, and G [length2] is the area of a geometrical cross
section of the particle in a plane perpendicular to the
direction of the incident light (i.e. the particle shadow).
The scattering efficiency may assume values greater than
unity (which conflicts with the traditionally accepted
meaning of this term, implying that its maximum value is
unity), i.e. a particle may scatter from the incident beam
more light power that falls on its geometrical cross section.](https://image.slidesharecdn.com/phy-addnofangmomentumslatersdeter-140719015942-phpapp02/75/Phy-addn-of-ang-momentum-slaters-deter-pep-29-2048.jpg)