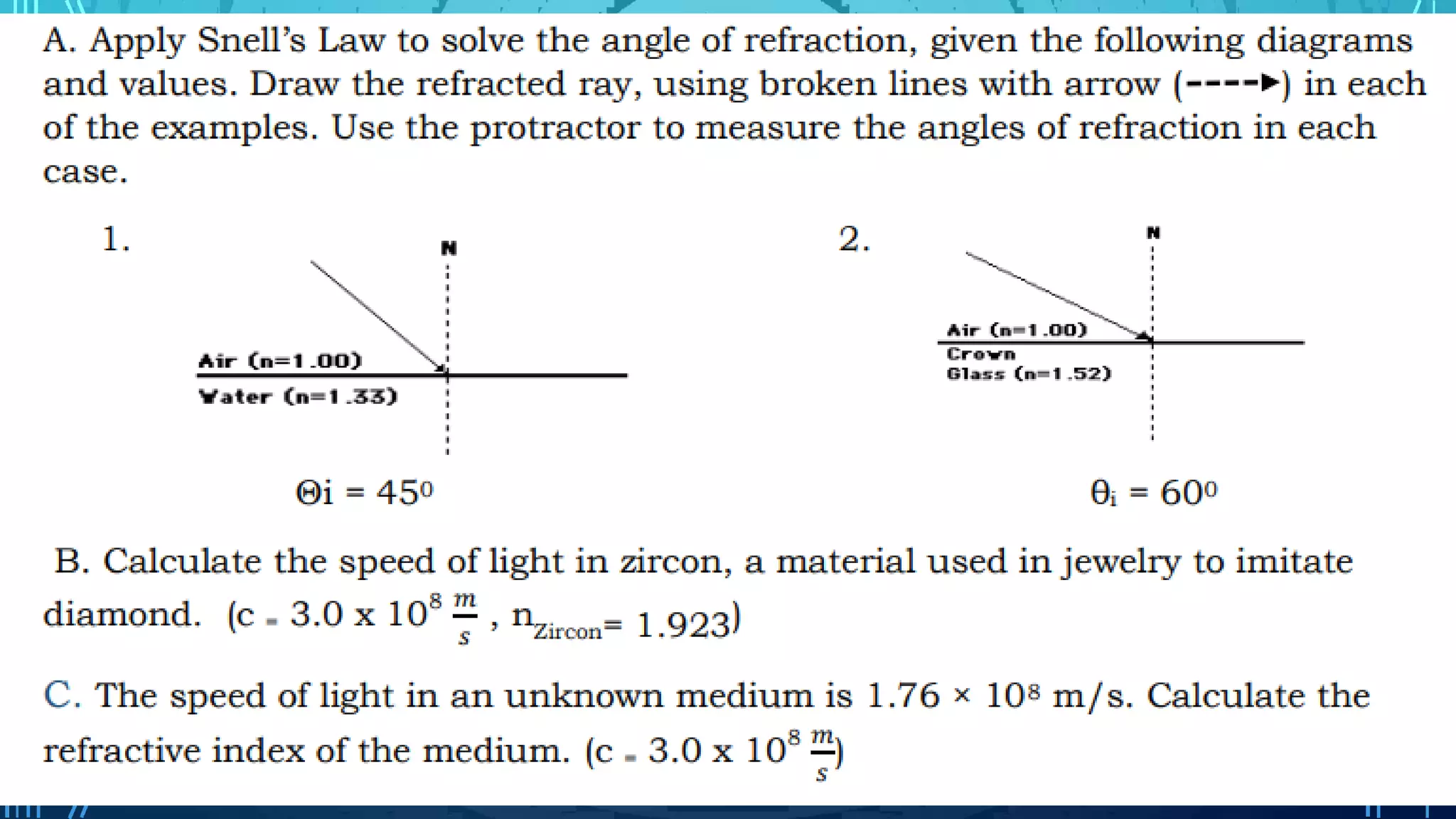
The document discusses the photon theory of light and its history. It describes Galileo and Roemer's early attempts to measure the speed of light and how Roemer observed Io to determine the speed was finite. It introduces photons as elementary particles that have properties of waves and particles, with a constant speed of 3x10^8 m/s. Einstein helped revive the particle theory of light and introduced the photoelectric effect to explain light's interaction with matter.