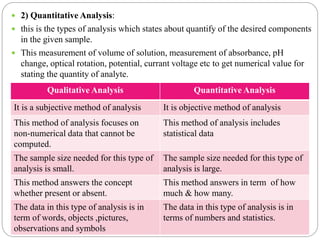
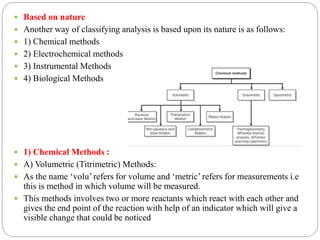
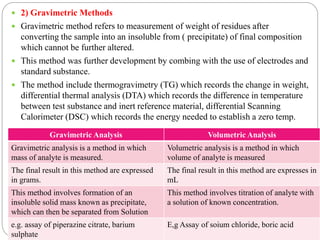
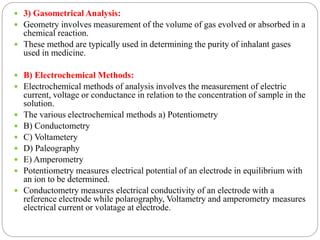
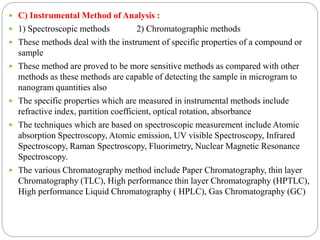
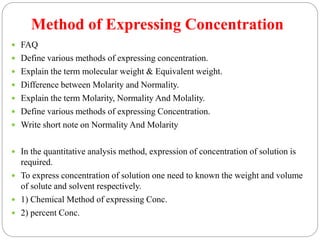
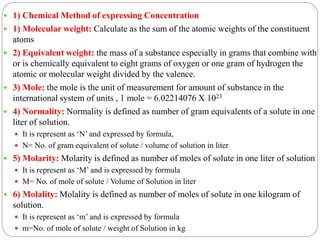
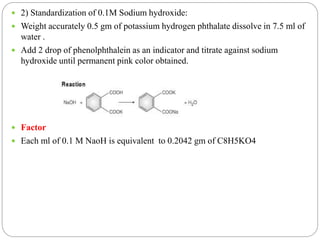
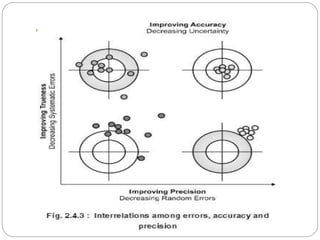
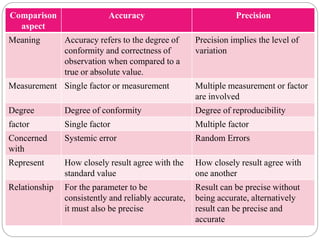
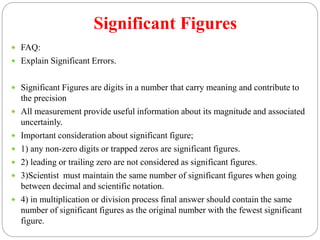
This document discusses pharmaceutical analysis and errors. It begins by defining pharmaceutical analysis as methods for identification, quantitation, and purification of samples. It describes the scope of pharmaceutical analysis, which includes identifying and analyzing active pharmaceutical ingredients, determining drug stability and efficacy, and mixture analysis. Various techniques of analysis are also discussed, including qualitative and quantitative methods, as well as volumetric, gravimetric, electrochemical, instrumental, and biological techniques. Methods for expressing concentration like molarity, normality, and percentage are also defined. The document concludes by discussing primary and secondary standards used in preparing standardized solutions.