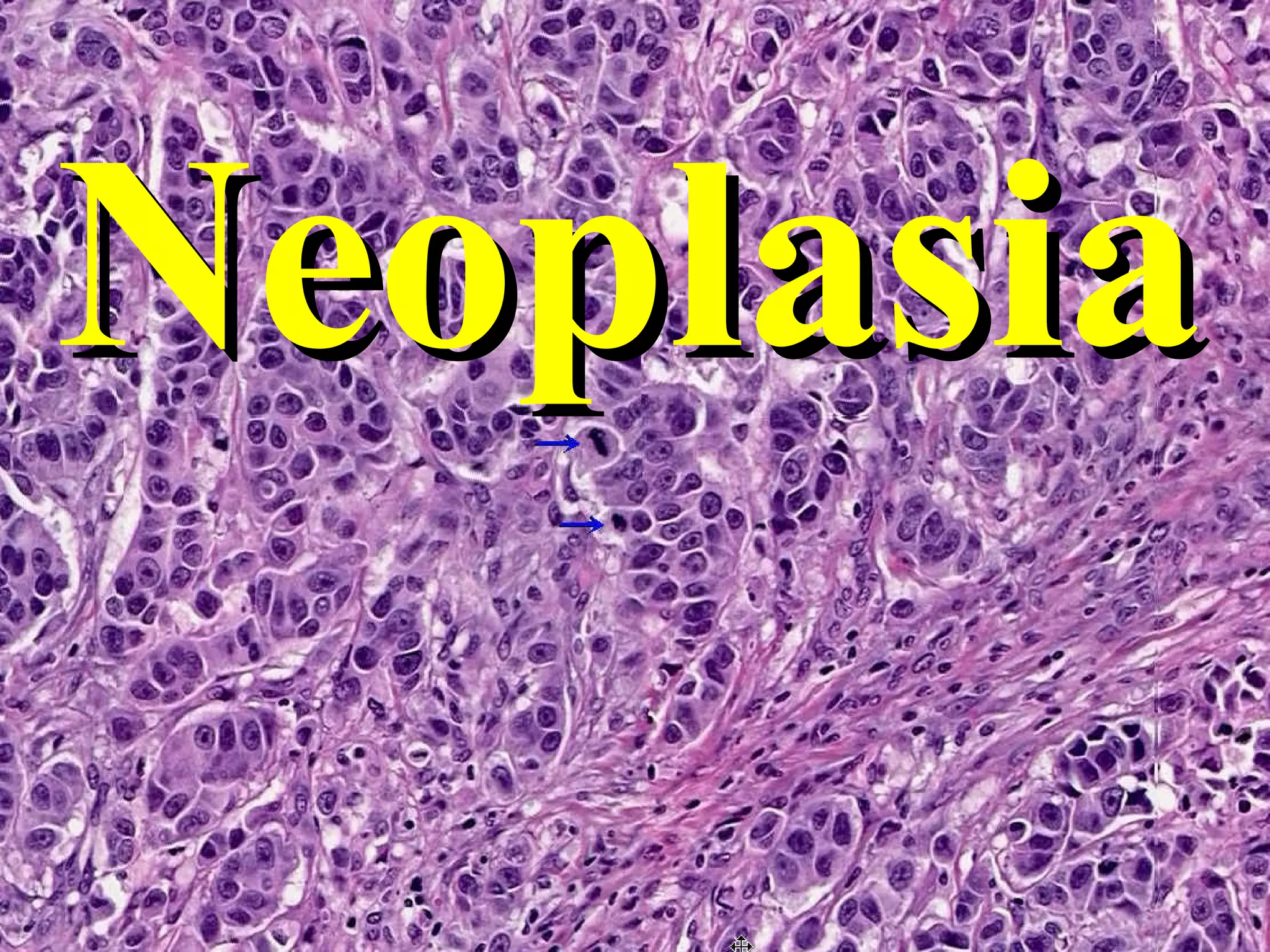
This document provides an overview of neoplasia (tumors), including definitions, nomenclature used to classify benign and malignant tumors, the biology of tumor growth, epidemiology, molecular basis of cancer and carcinogenesis. It discusses the natural history of malignant tumors from malignant change to growth, invasion and metastasis. Key concepts covered include differentiation, dysplasia, tumor growth rates, features that distinguish benign from malignant tumors, predisposing geographic, environmental and genetic factors for cancer development.