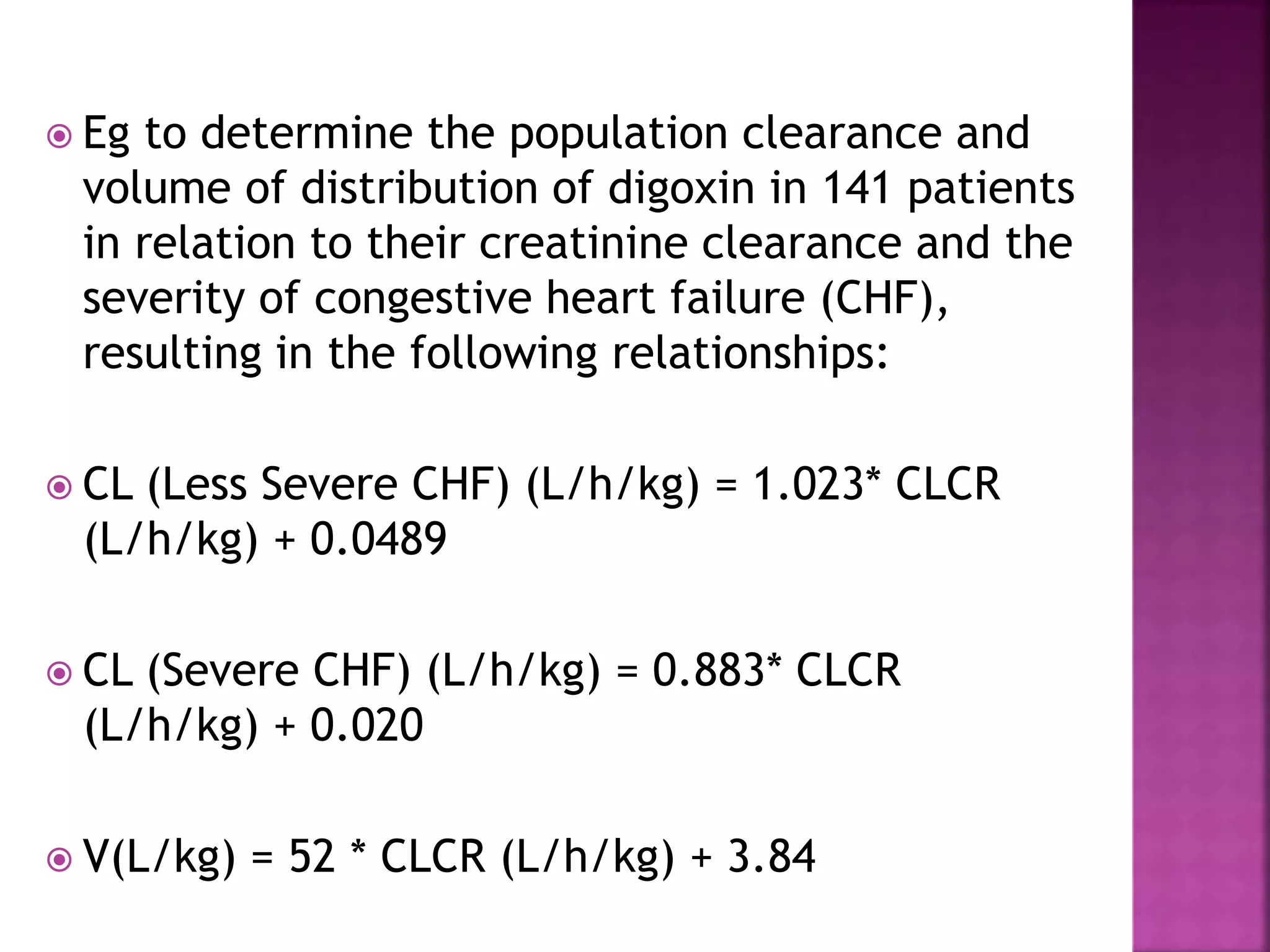
Nomograms are used to estimate pharmacokinetic parameters for individualized drug dosing based on patient characteristics such as age and weight, particularly when specific patient data is unavailable. These predictive tools are derived from population data and assist in initial dosage regimen design, patient-specific parameter estimation, and dosage adjustment as needed. Two primary methods for obtaining population pharmacokinetic data are the traditional two-stage method, which analyzes drug clearance in controlled groups, and the nonlinear mixed effects model that aggregates data from varied patient samples.