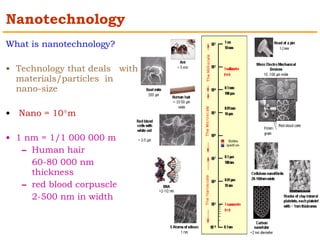
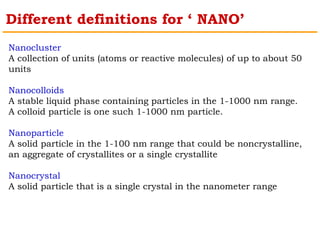
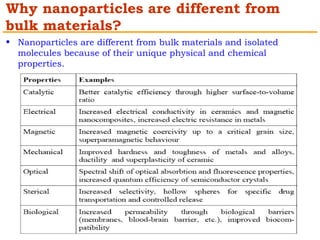
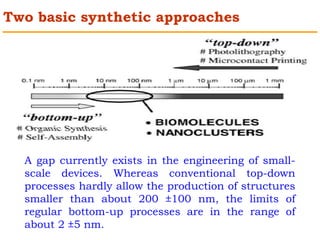
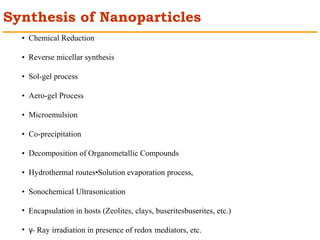
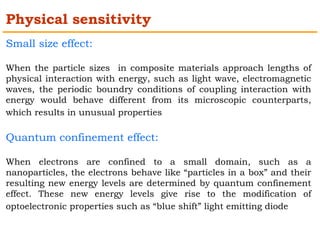
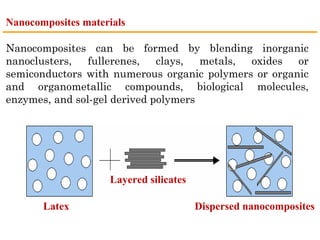
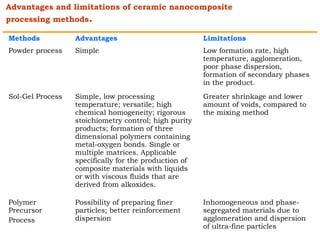
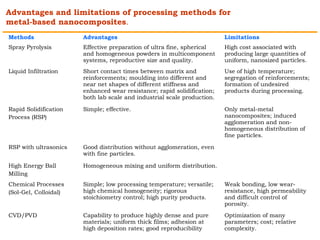
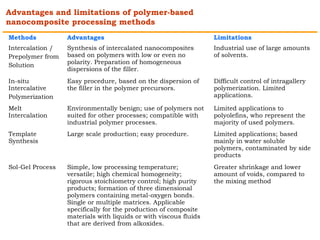
This document provides an overview of nanocomposite materials. It defines nanocomposites as materials with at least one component that has dimensions between 1-100 nm. Nanocomposites consist of inorganic or organic nanoparticles embedded in a matrix. They exhibit enhanced and unique properties compared to bulk materials due to quantum effects and high surface area. The document discusses various synthesis methods for nanomaterials and nanocomposites, as well as their advantages and limitations.