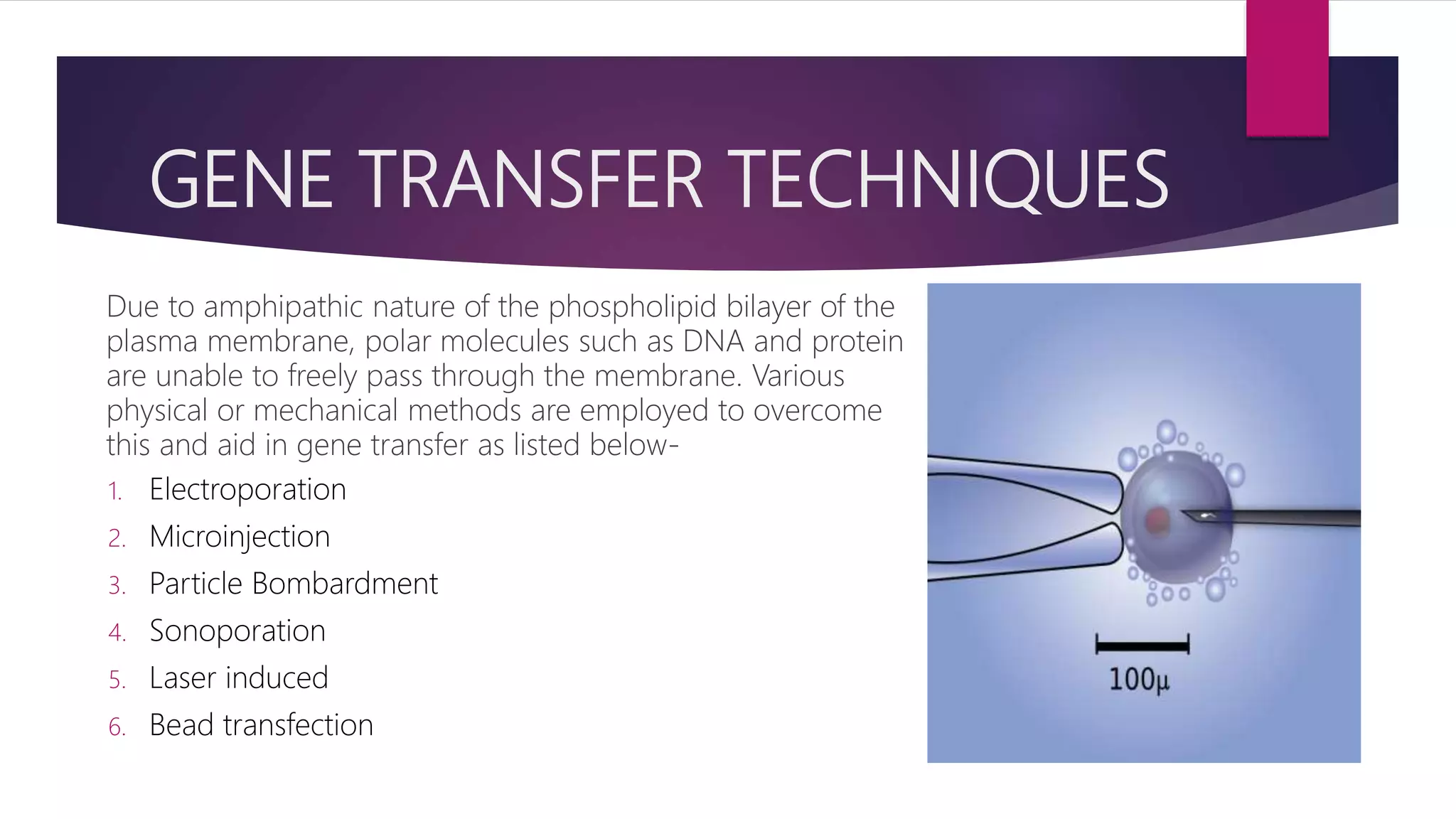
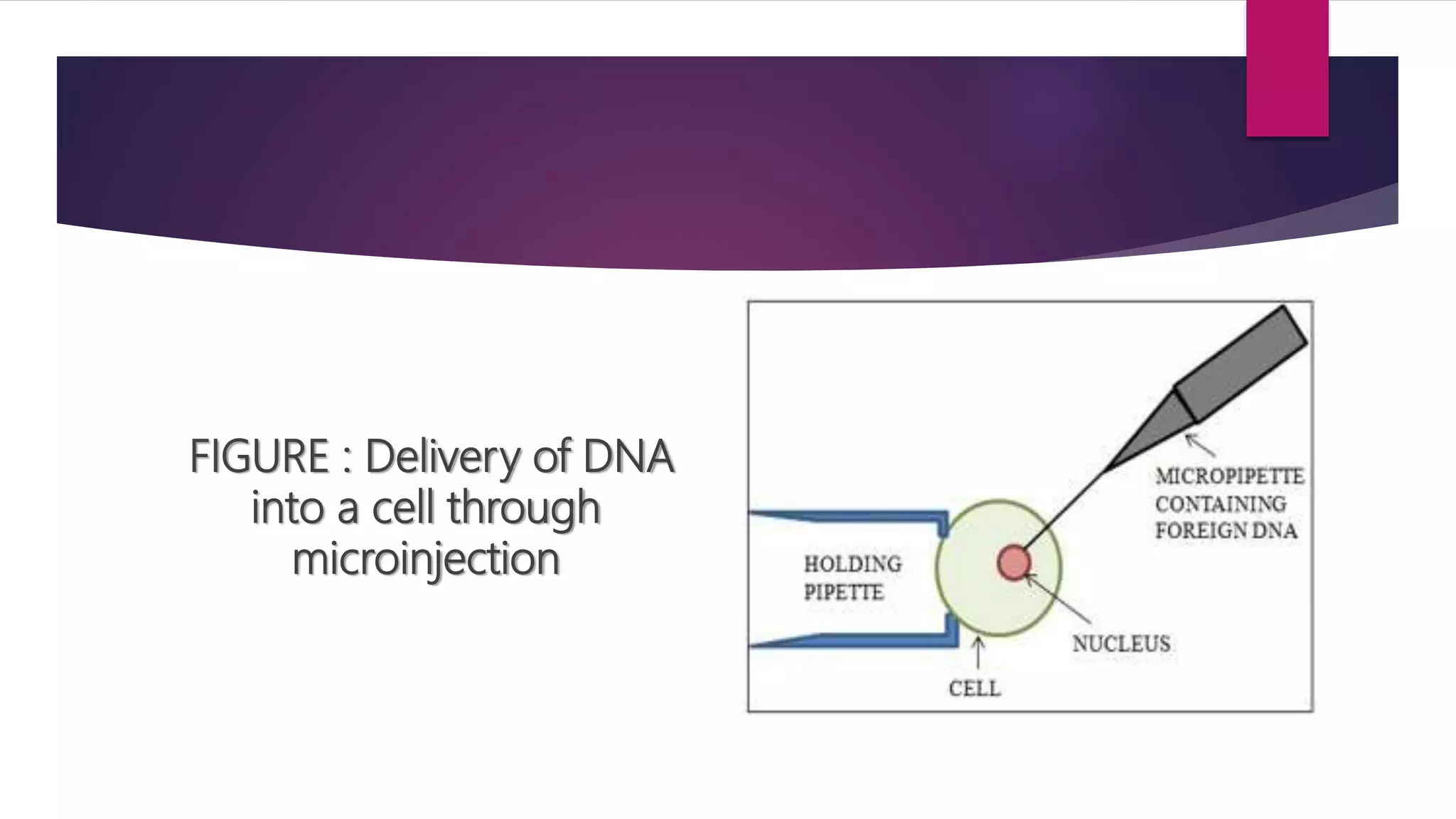
Microinjection is a gene transfer technique where DNA is directly injected into cells using a fine glass micropipette. It is highly efficient at the individual cell level and was originally used for transfecting hard-to-transfect cells. The procedure involves holding a cell using one pipette while another pipette is used to inject DNA into the cell's cytoplasm or nucleus. It allows for stable transfection efficiencies of around 20% and is used to generate transgenic animals by injecting DNA into oocytes, eggs or embryos. However, it is time-consuming and can only be done for a small number of cells.