Matter can be summarized as follows:
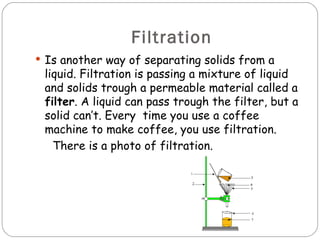
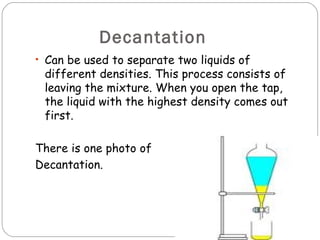
Matter is anything that occupies space and has mass. It exists in three states - solid, liquid, and gas. Matter changes between these states through processes like fusion, condensation, evaporation, and solidification. Matter can be classified as pure substances or mixtures. Mixtures contain more than one substance and can be separated using methods like evaporation, distillation, filtration, and decantation. Changes to matter can be physical, changing its shape or size, or chemical, changing its composition.