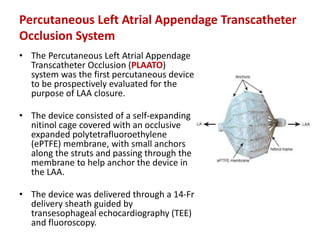
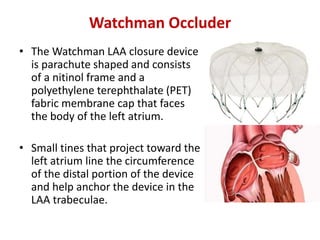
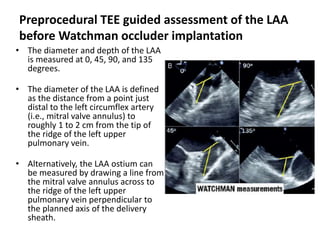
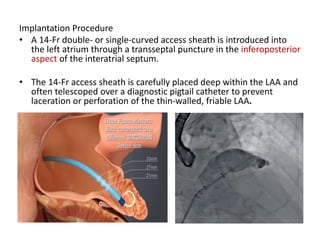
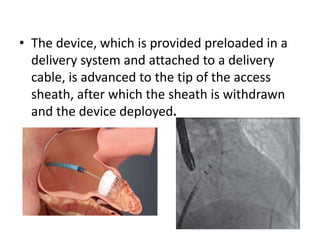
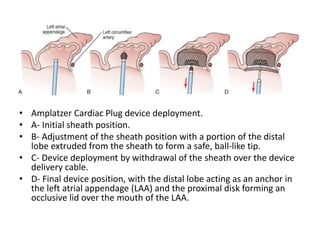
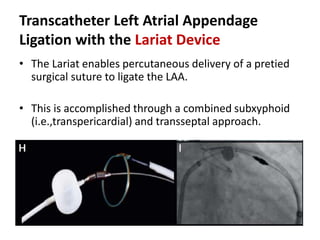
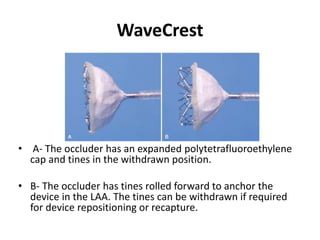
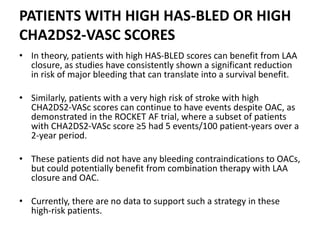
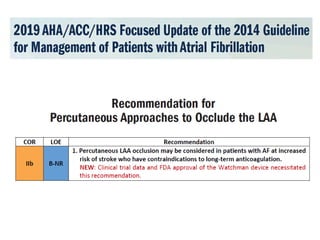
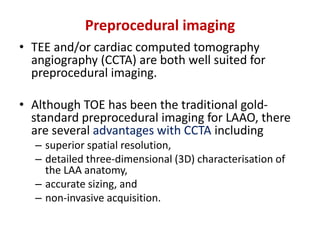
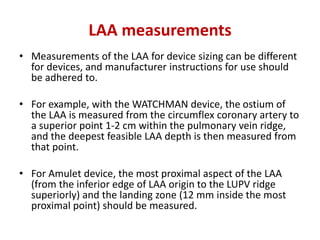
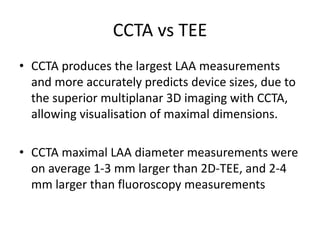
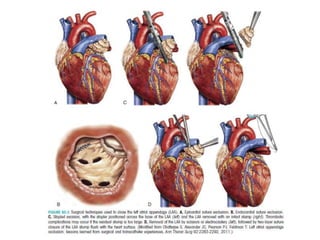
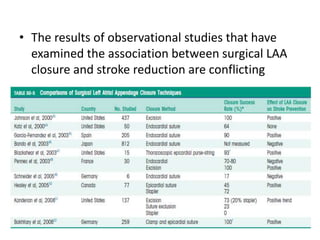
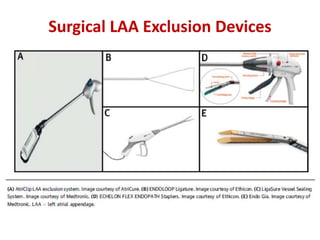
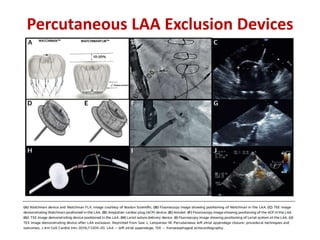
The left atrial appendage (LAA) is a remnant of the left atrium that can be a source of thrombus and stroke in patients with atrial fibrillation. Several percutaneous devices have been developed to occlude the LAA to prevent thrombus formation and reduce the risk of stroke, including the Watchman device. The Watchman is a nitinol frame covered with PET fabric that is implanted via transseptal puncture and deployed in the LAA orifice. Correct placement is confirmed using TEE and fluoroscopy to ensure the device is properly positioned, anchored, sized, and sealing the LAA opening.





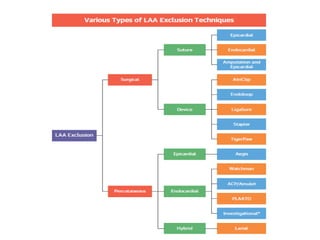



![• Current catheter-based devices for LAAO are based on three
different principles:
– Plug: endovascular delivery of a device lobe or umbrella
obstructing the neck of the LAA, thereby preventing blood flow
into the body of the LAA. LAA exclusion relies on
sealing/endocardialisation of the device lobe/umbrella.
• e.g. WATCHMANTM, WaveCrestVR
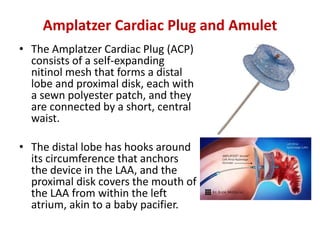
– Pacifier principle: endovascular delivery of a device with a lobe
or umbrella and an additional disc to seal the ostium of the LAA
from the left atrial side. LAA exclusion relies on
sealing/endocardialisation of the device lobe/umbrella
• e.g. AMPLATZERTM Cardiac Plug [ACP], AmuletTM and/or the sealing
disc (ACP, Amulet, Ultraseal, LAmbreTM.
– Ligation: LARIAT to snare and ligate the body of the LAA using an
endocardial and epicardial approach. LAA exclusion relies on
complete ligation of the neck of the LAA](https://image.slidesharecdn.com/leftatrialappendageclosure-200305160733/85/Left-atrial-appendage-closure-26-320.jpg)