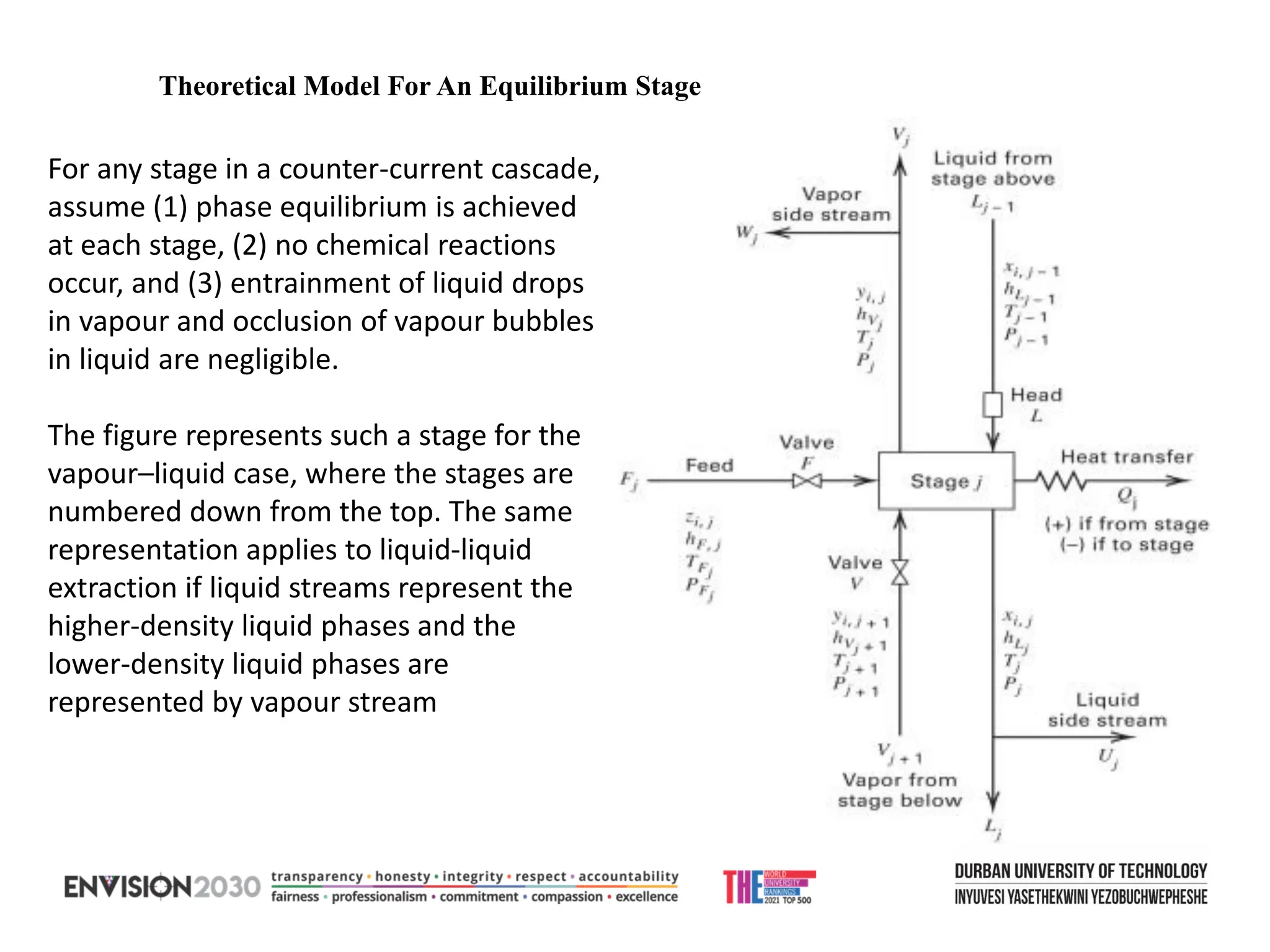
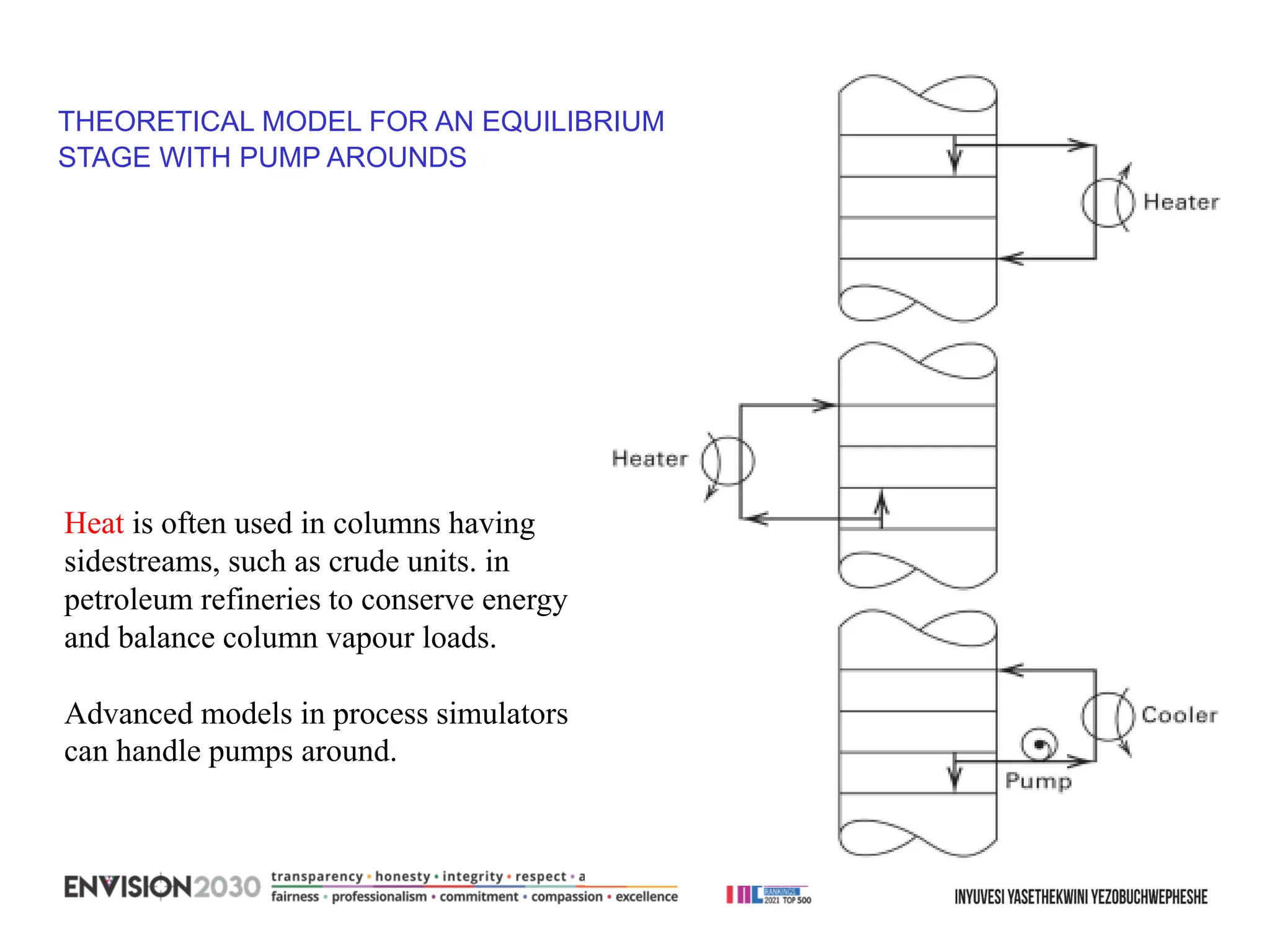
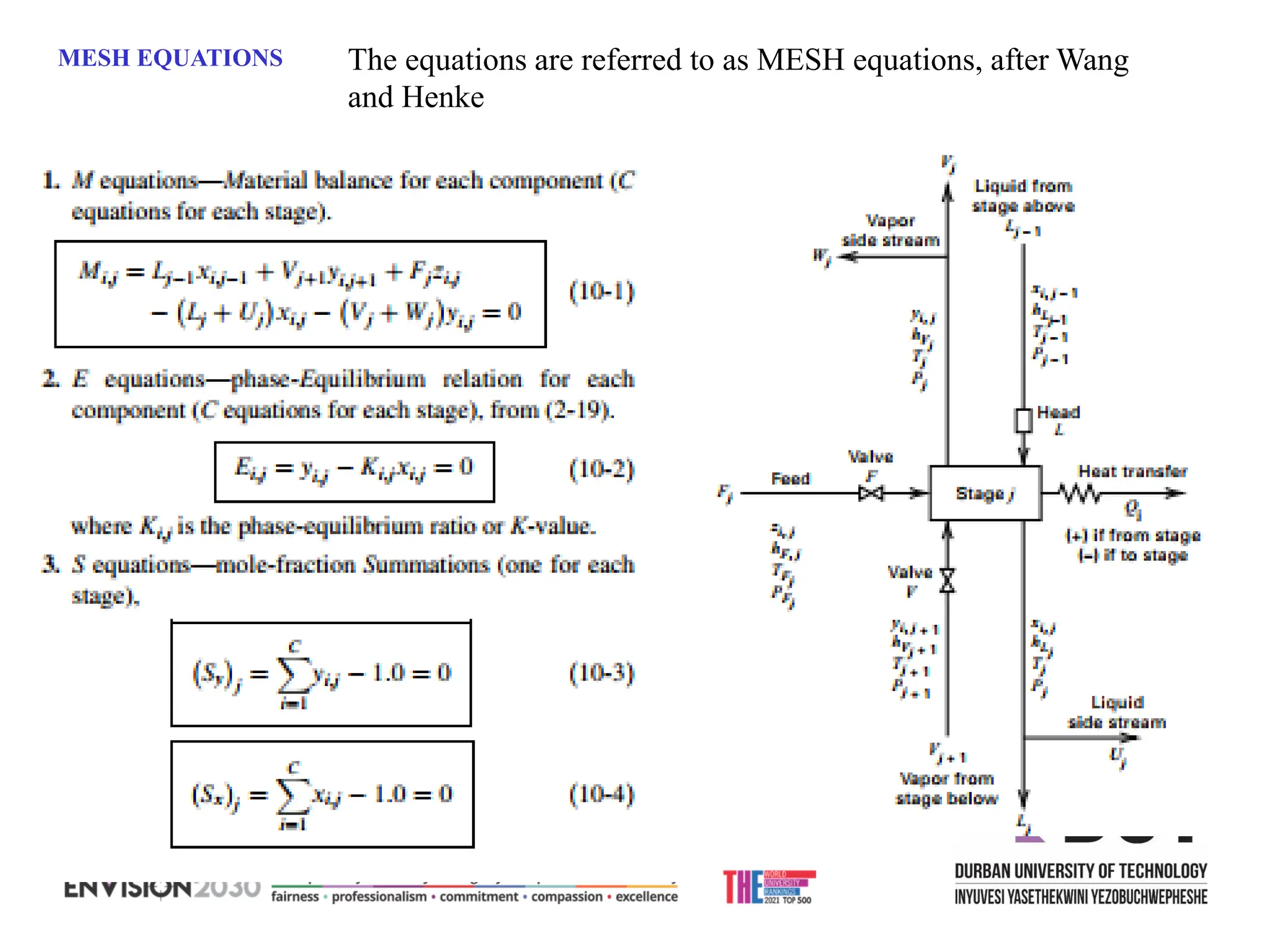
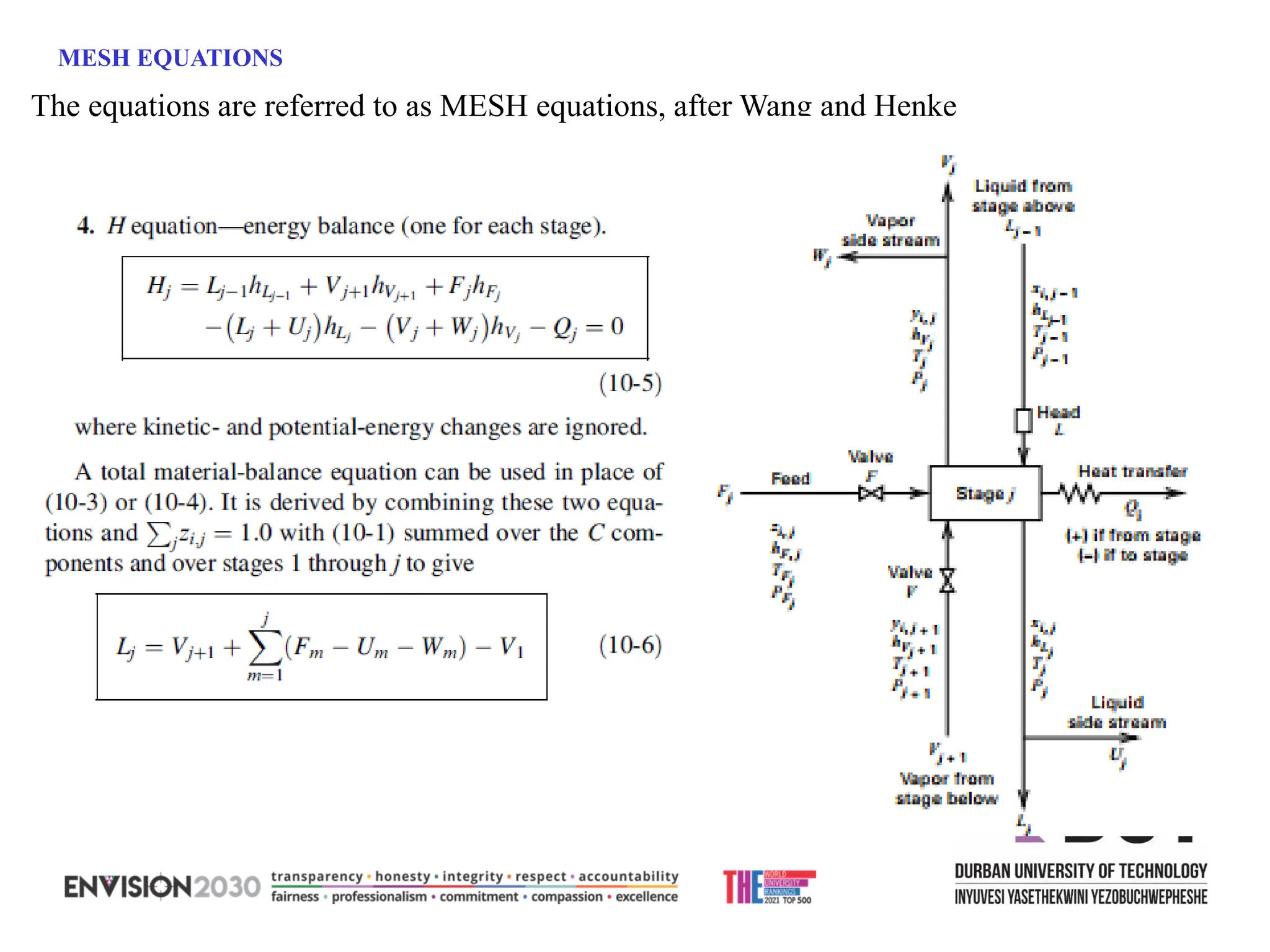
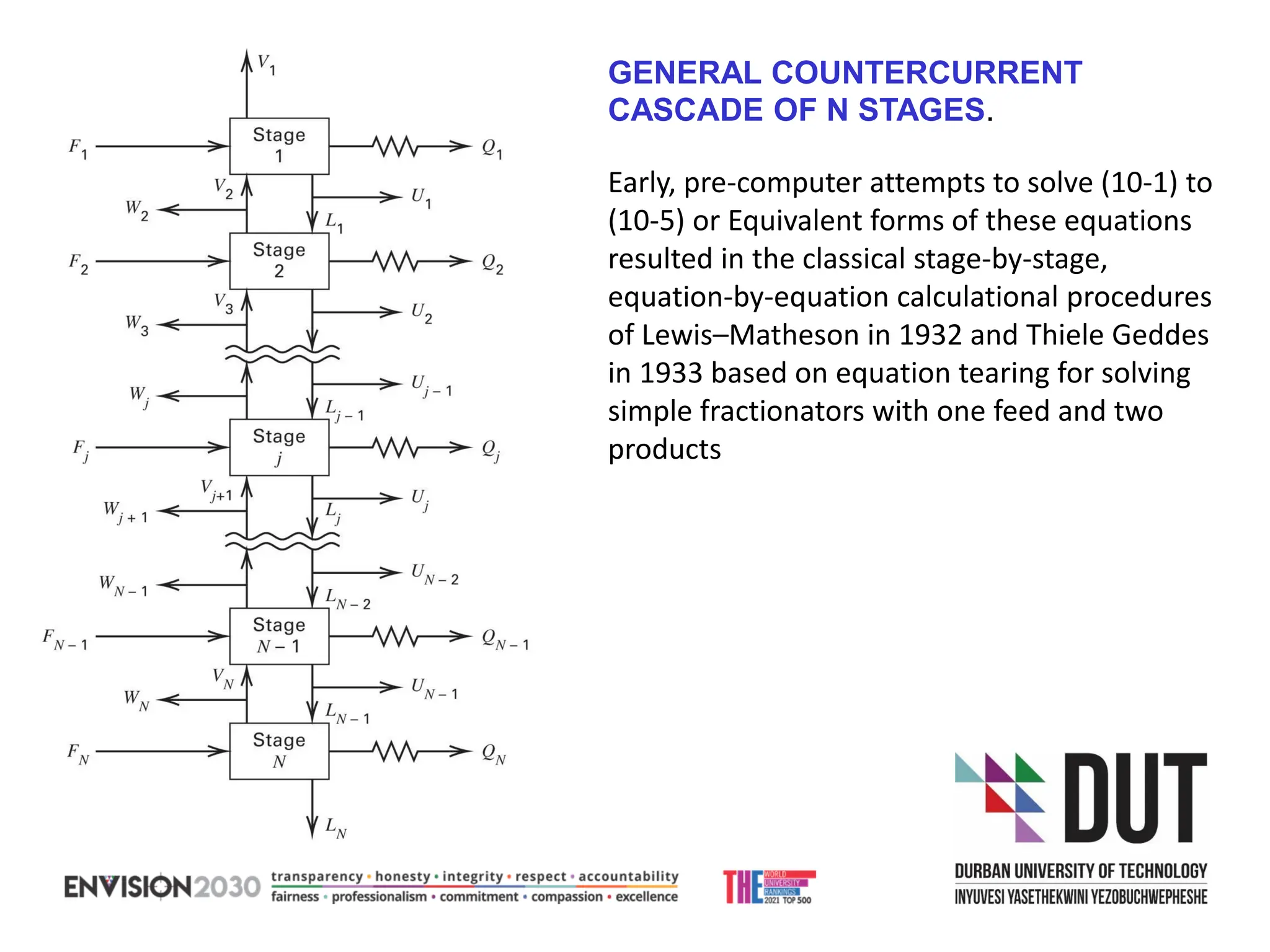
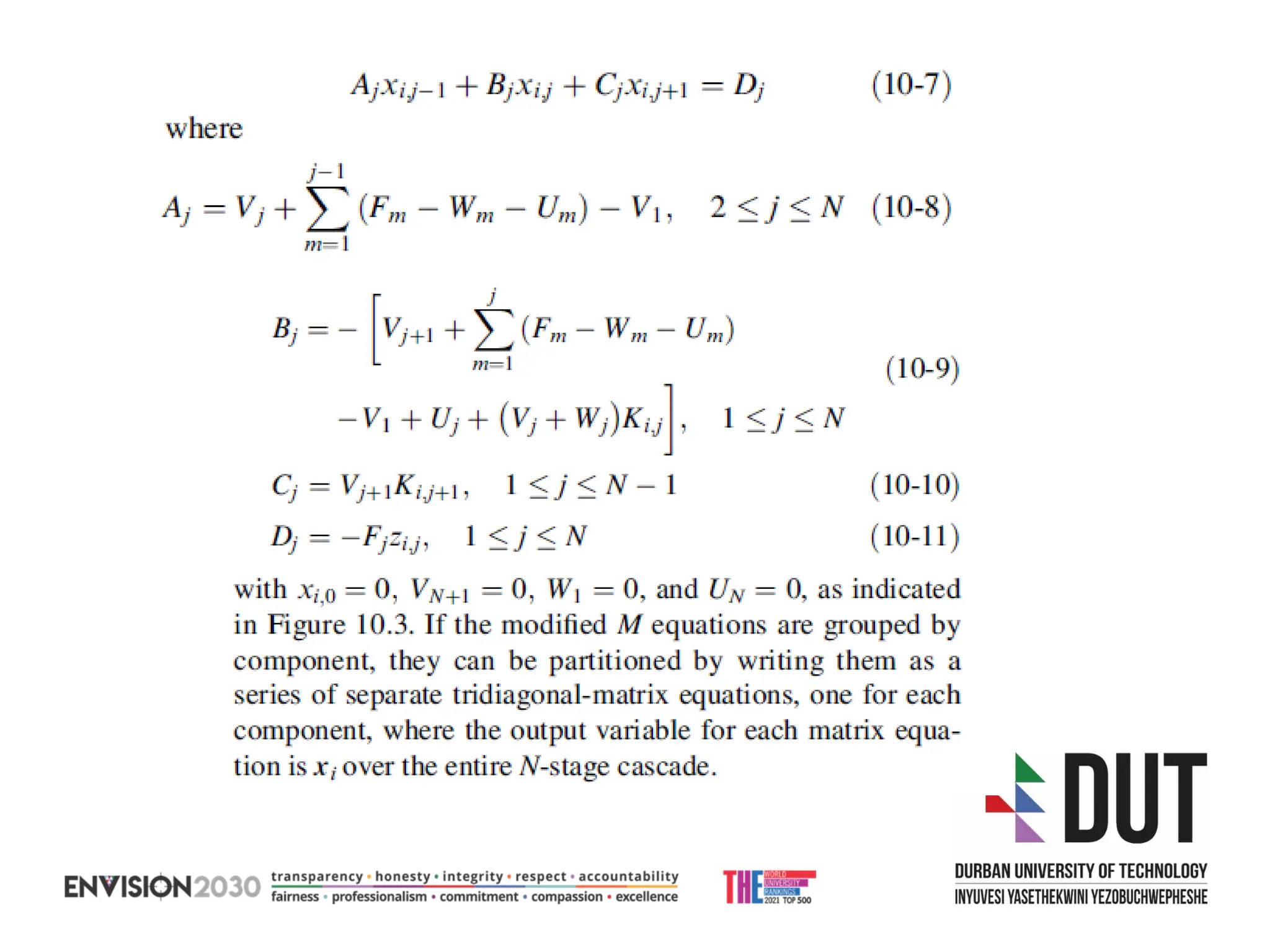
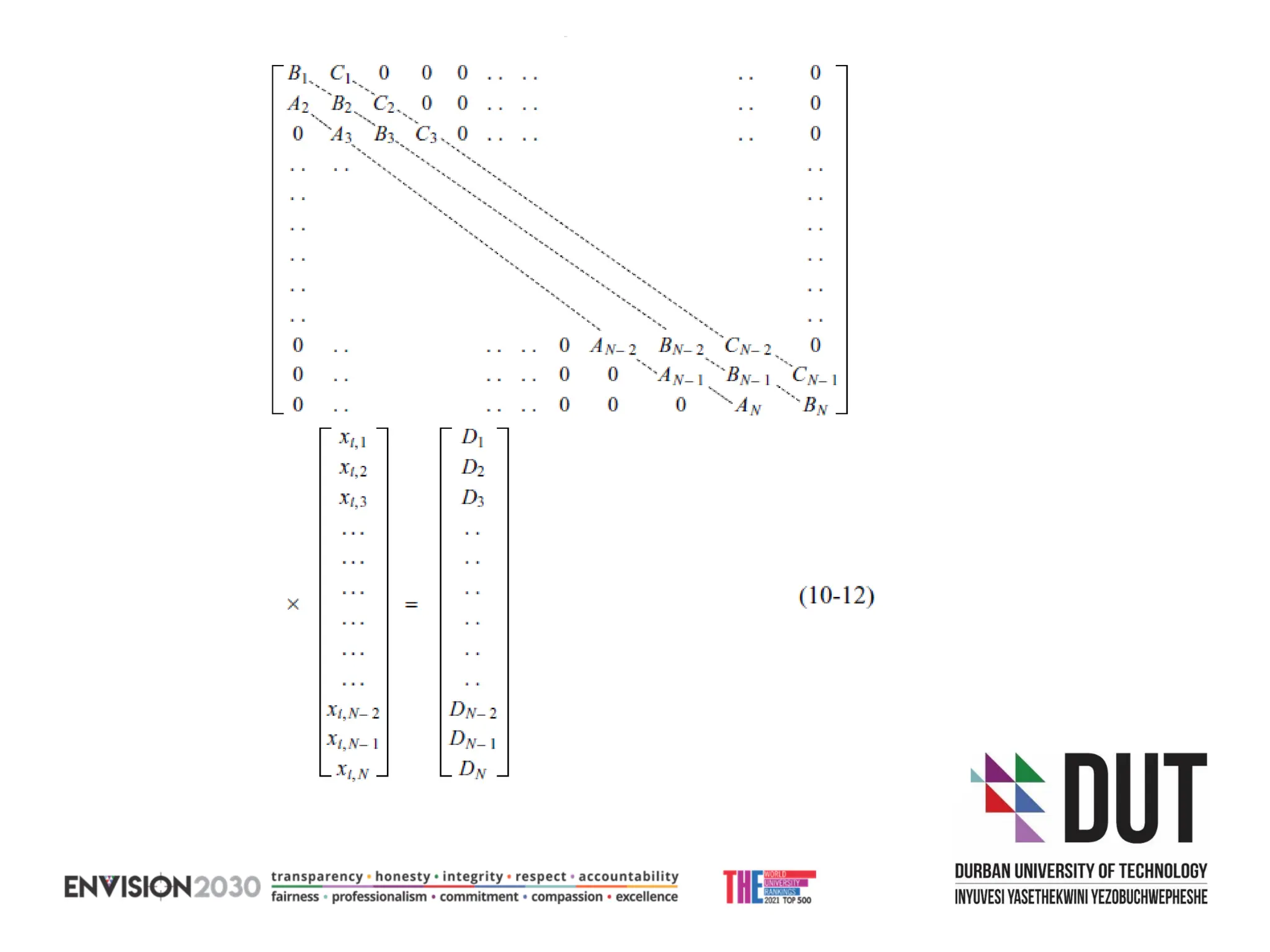
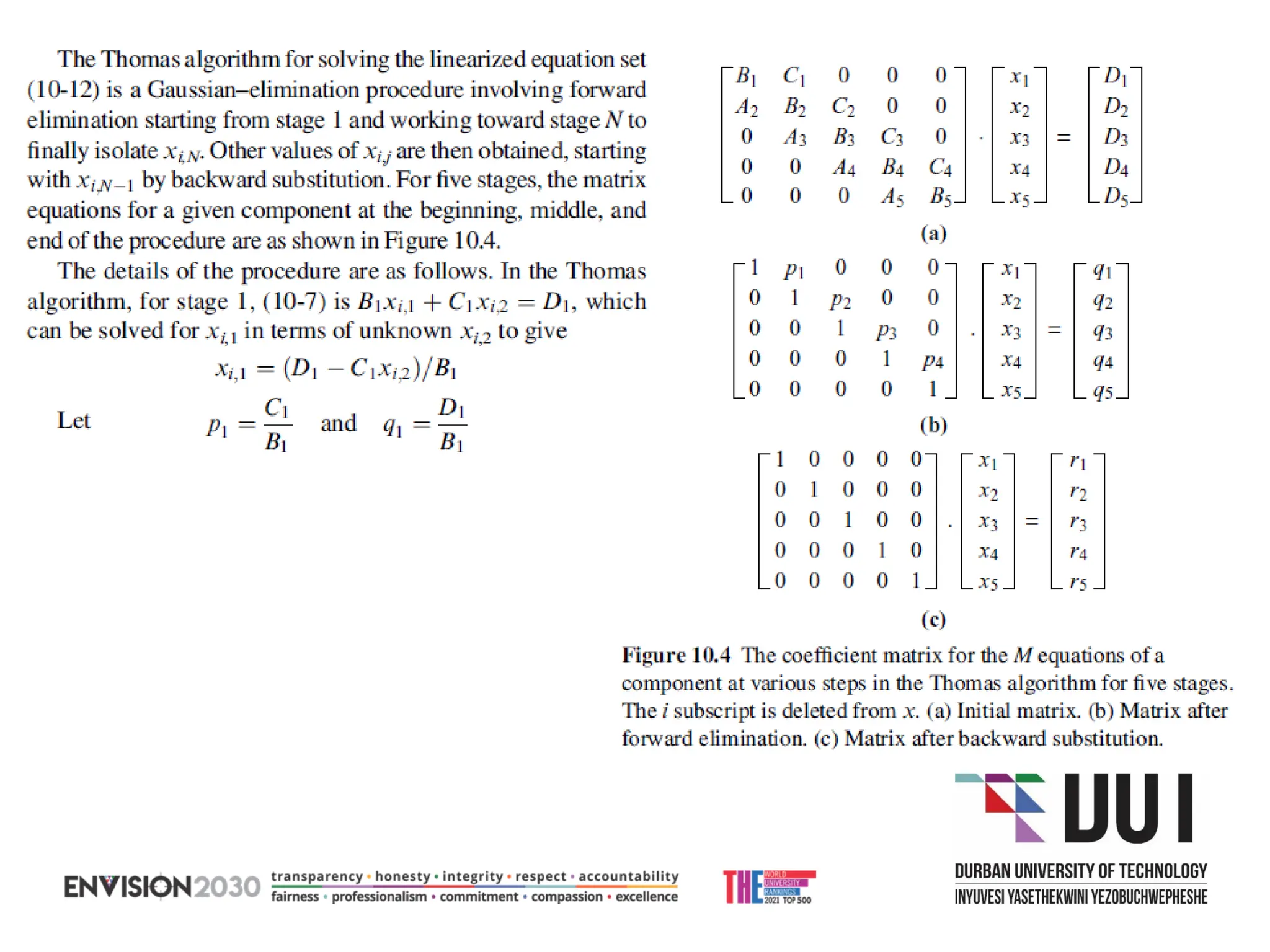
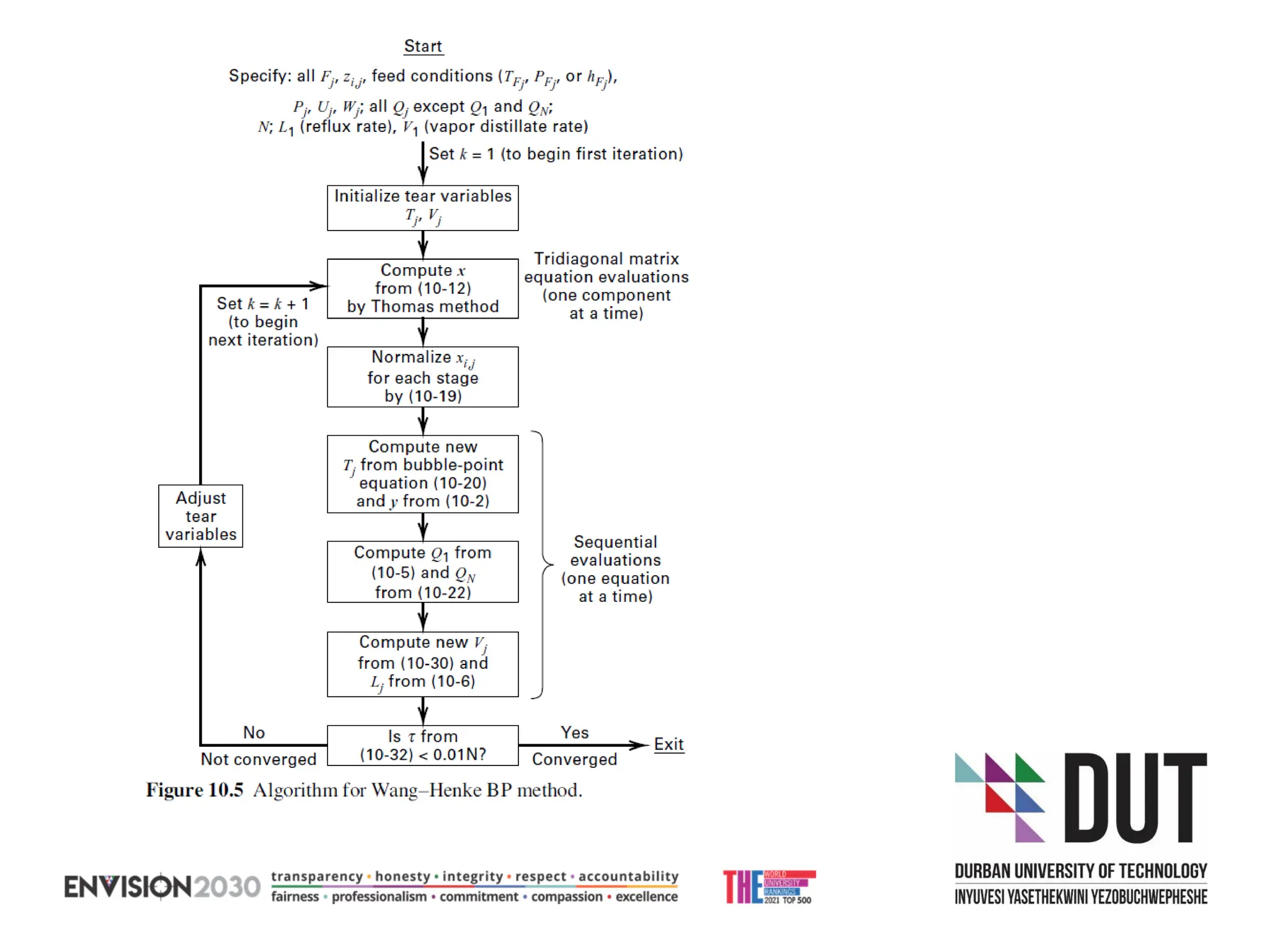
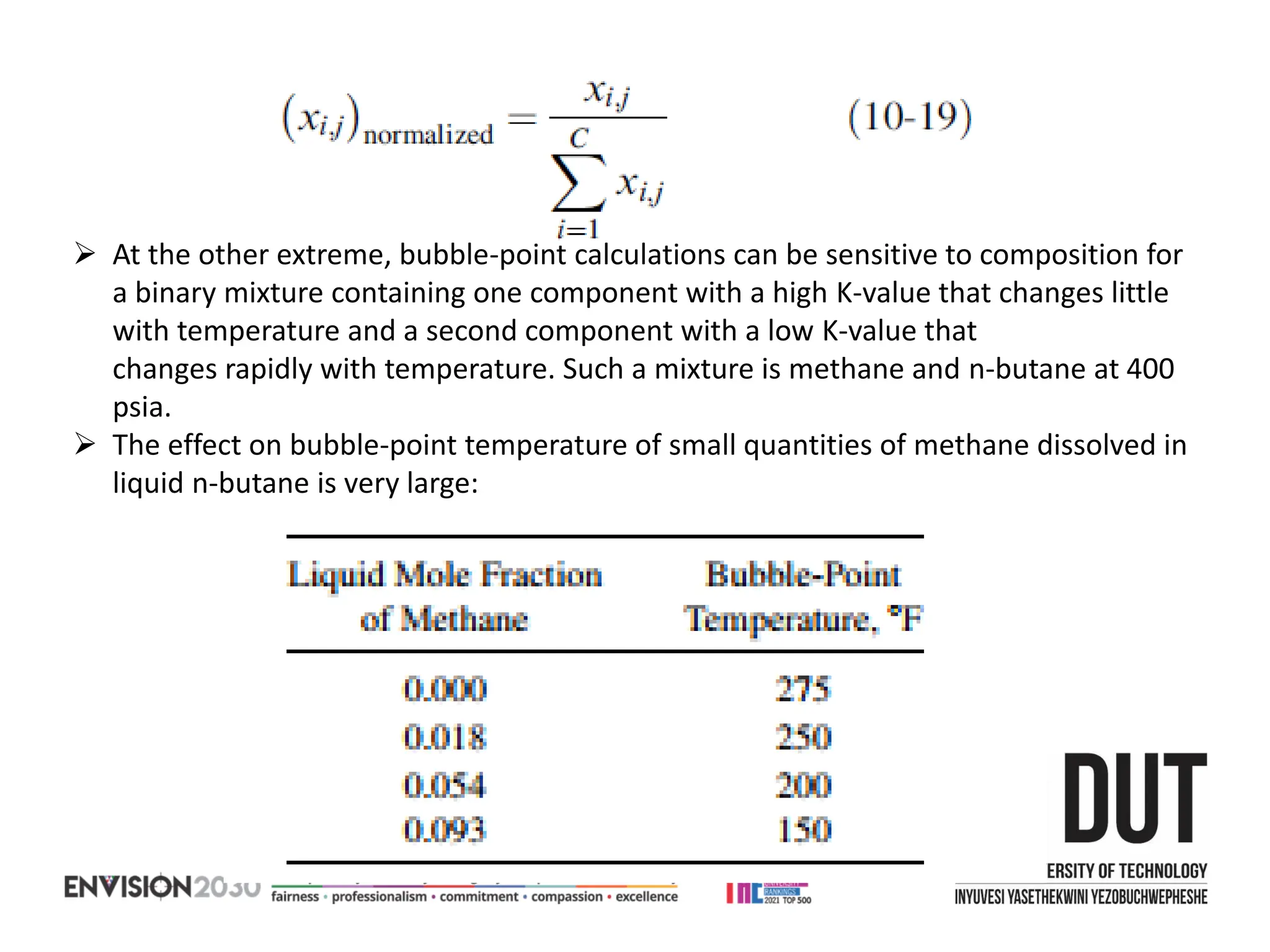
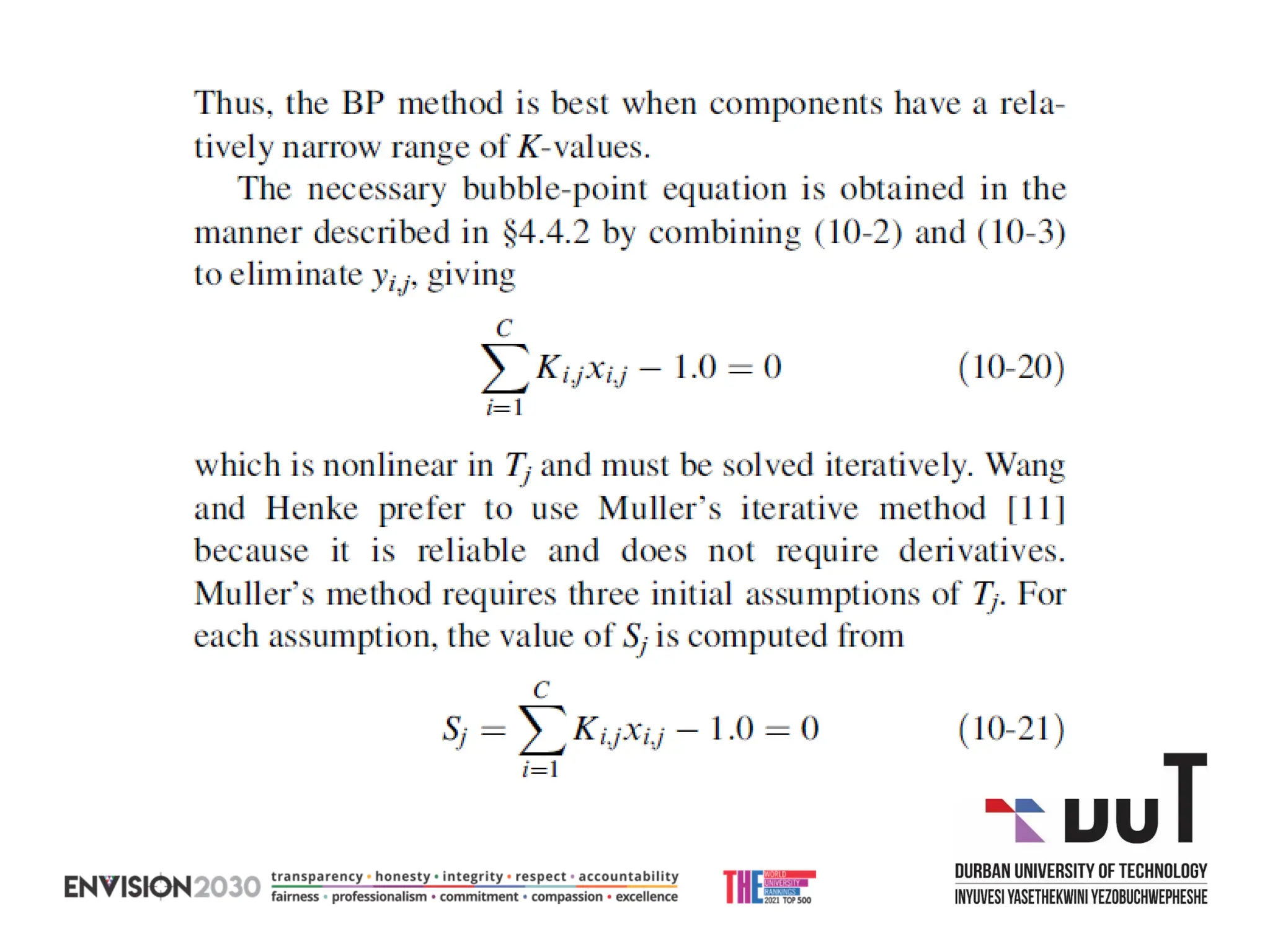
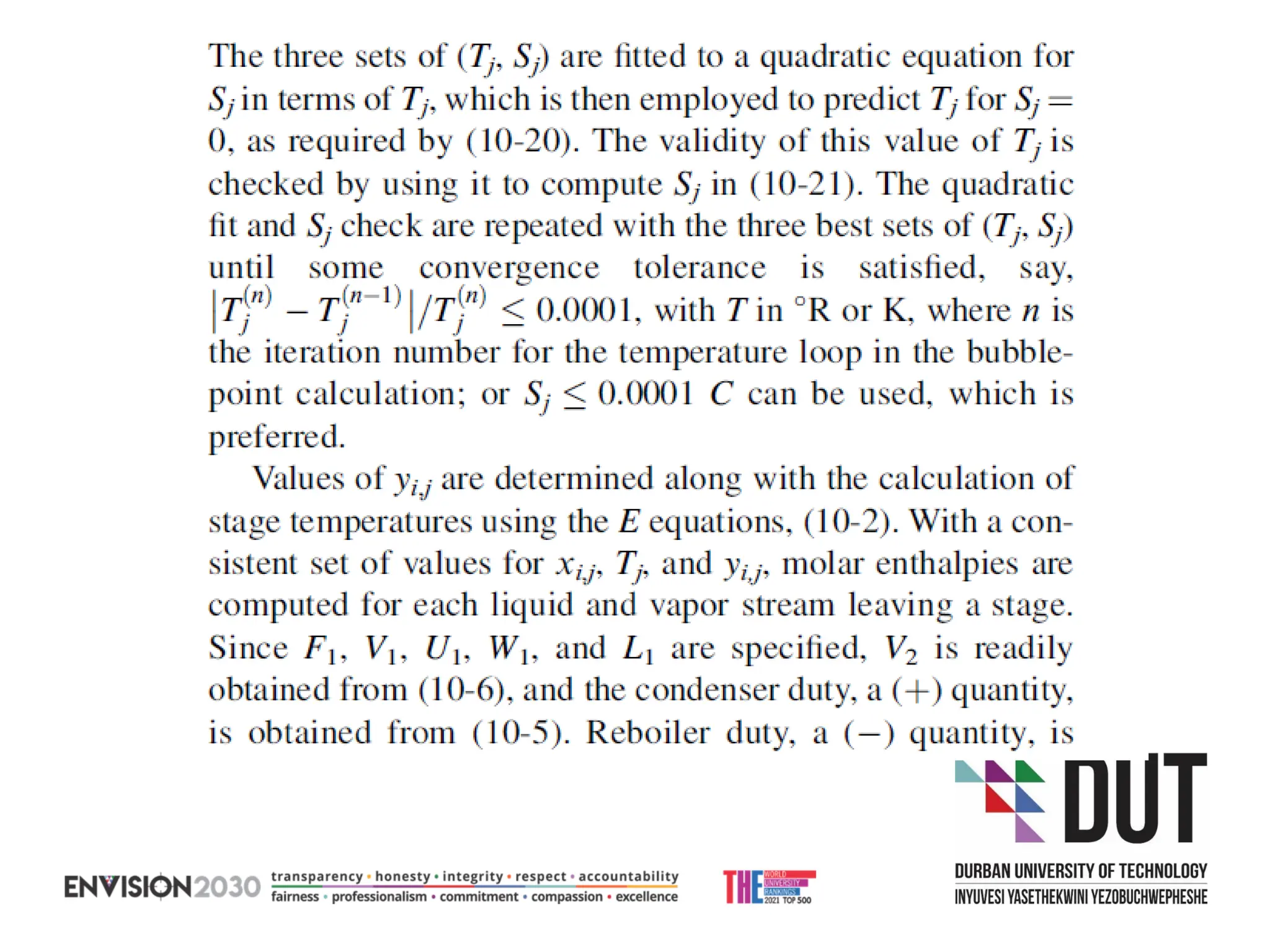
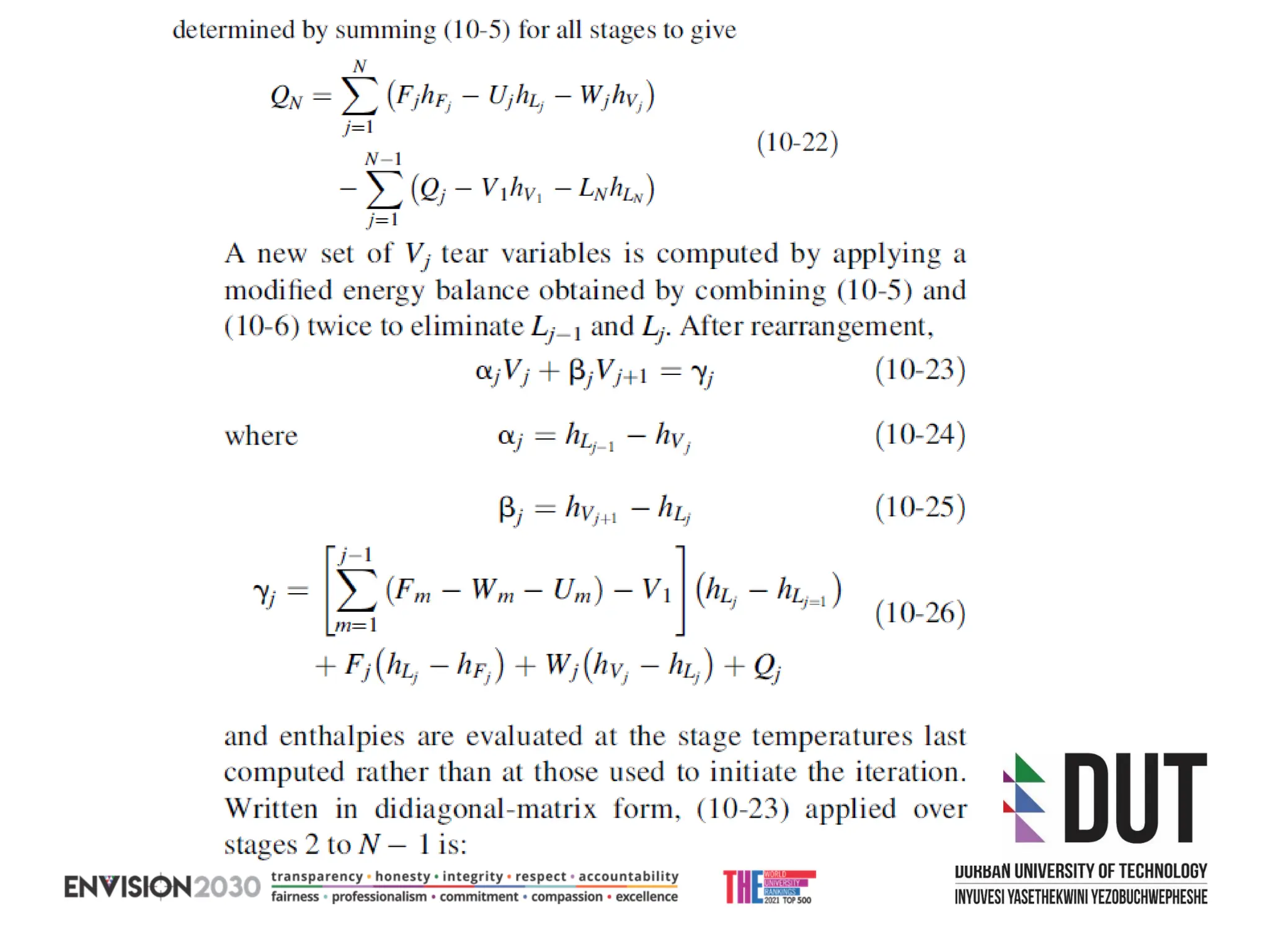
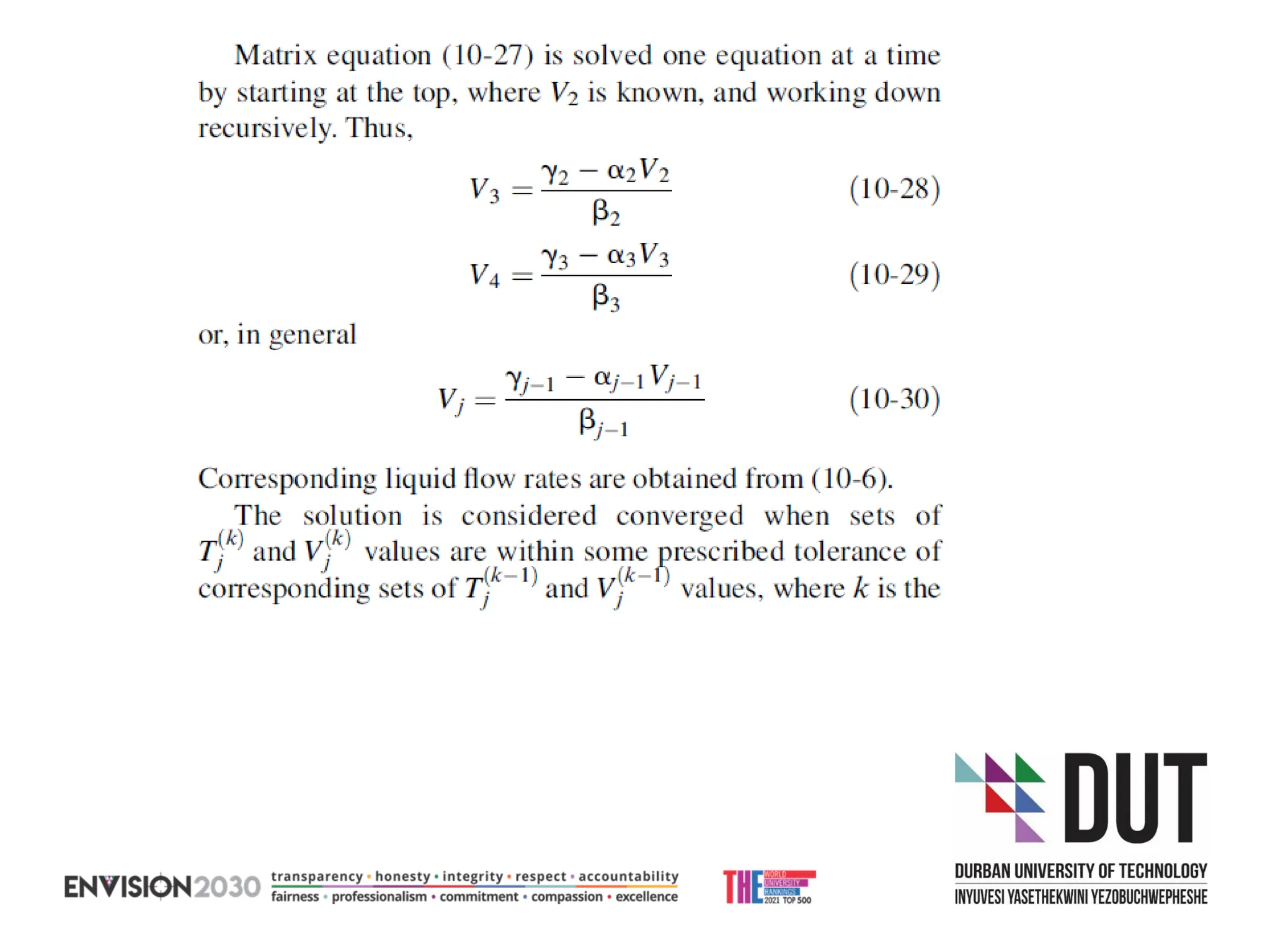
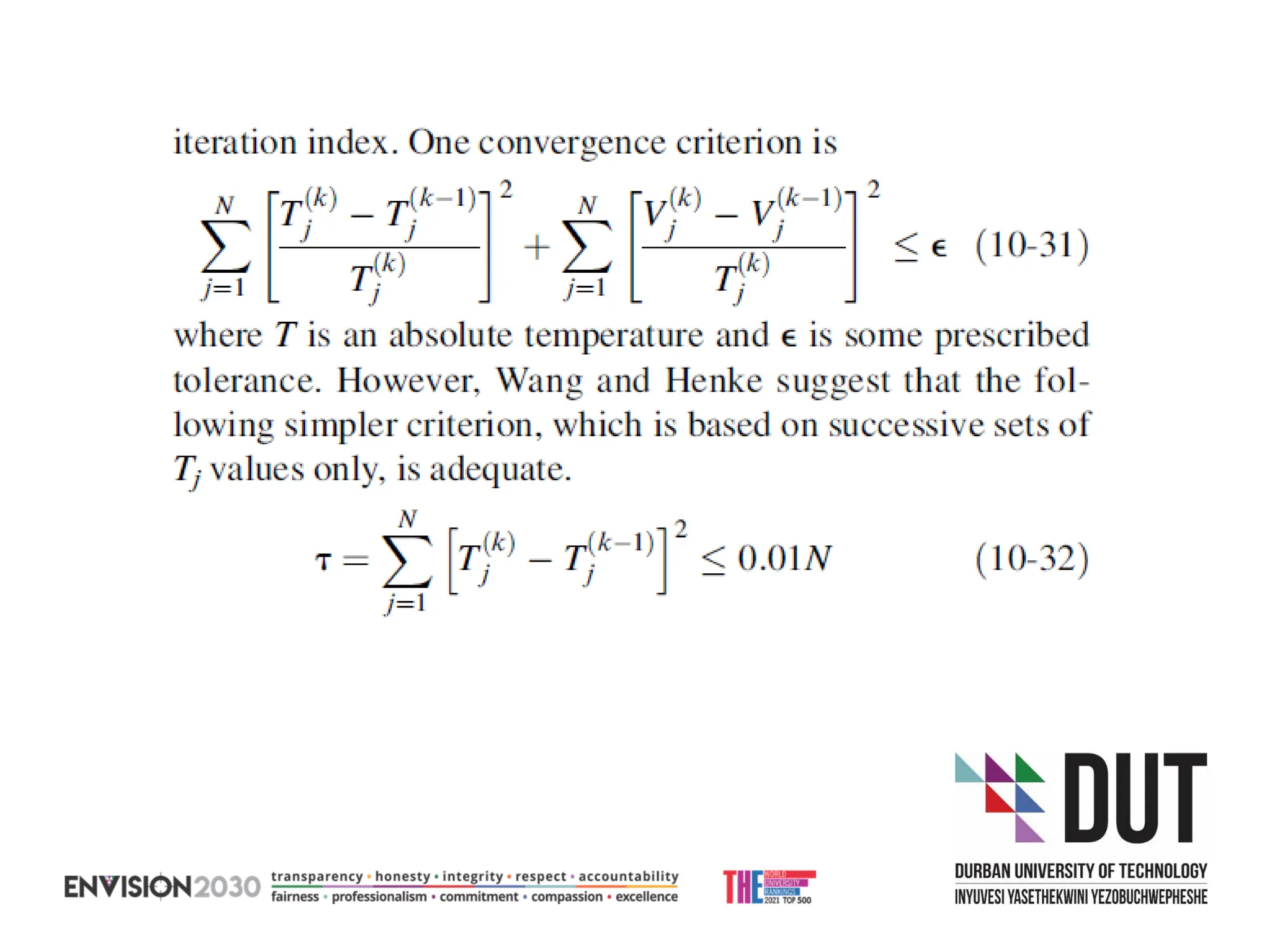
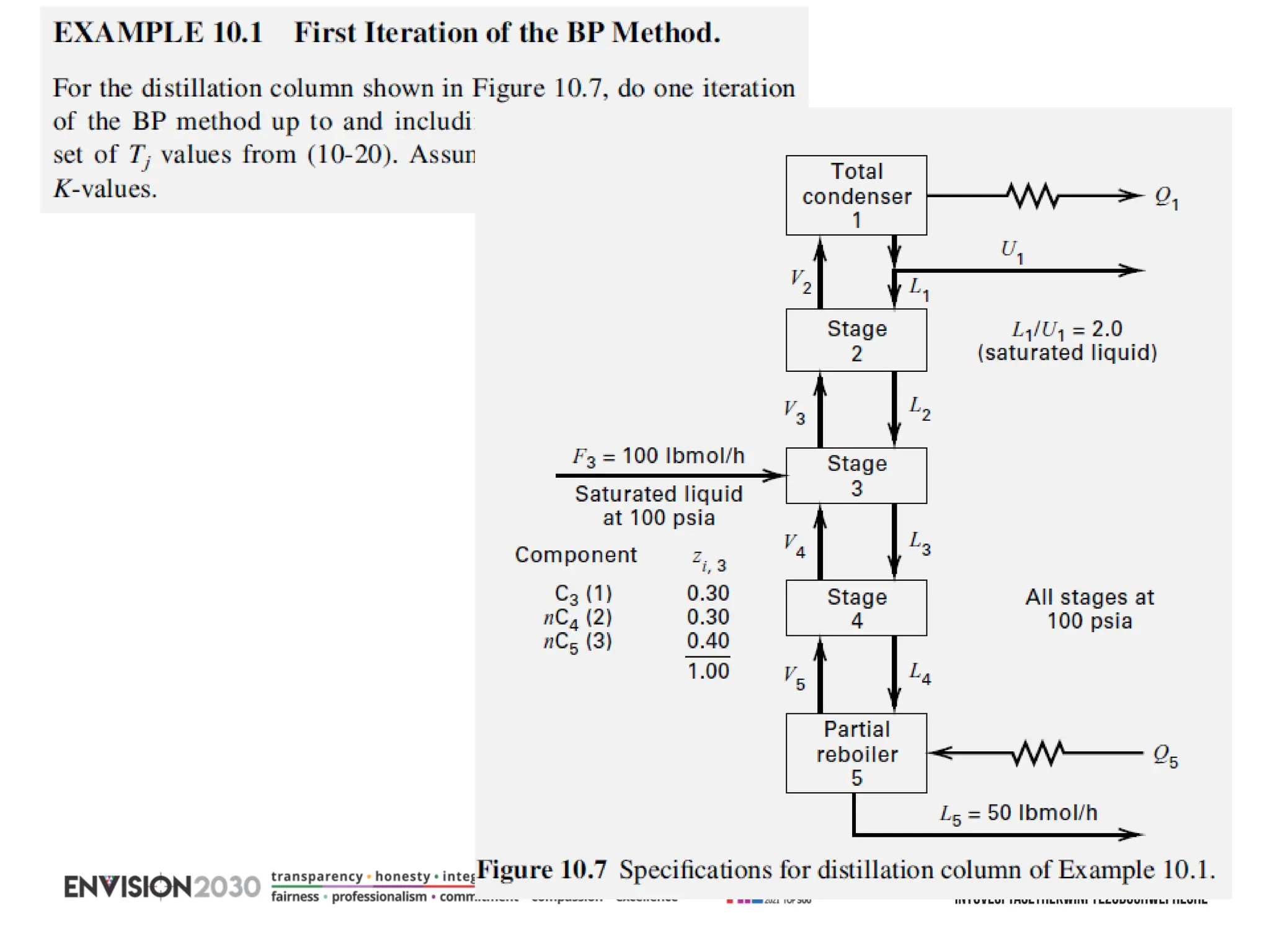
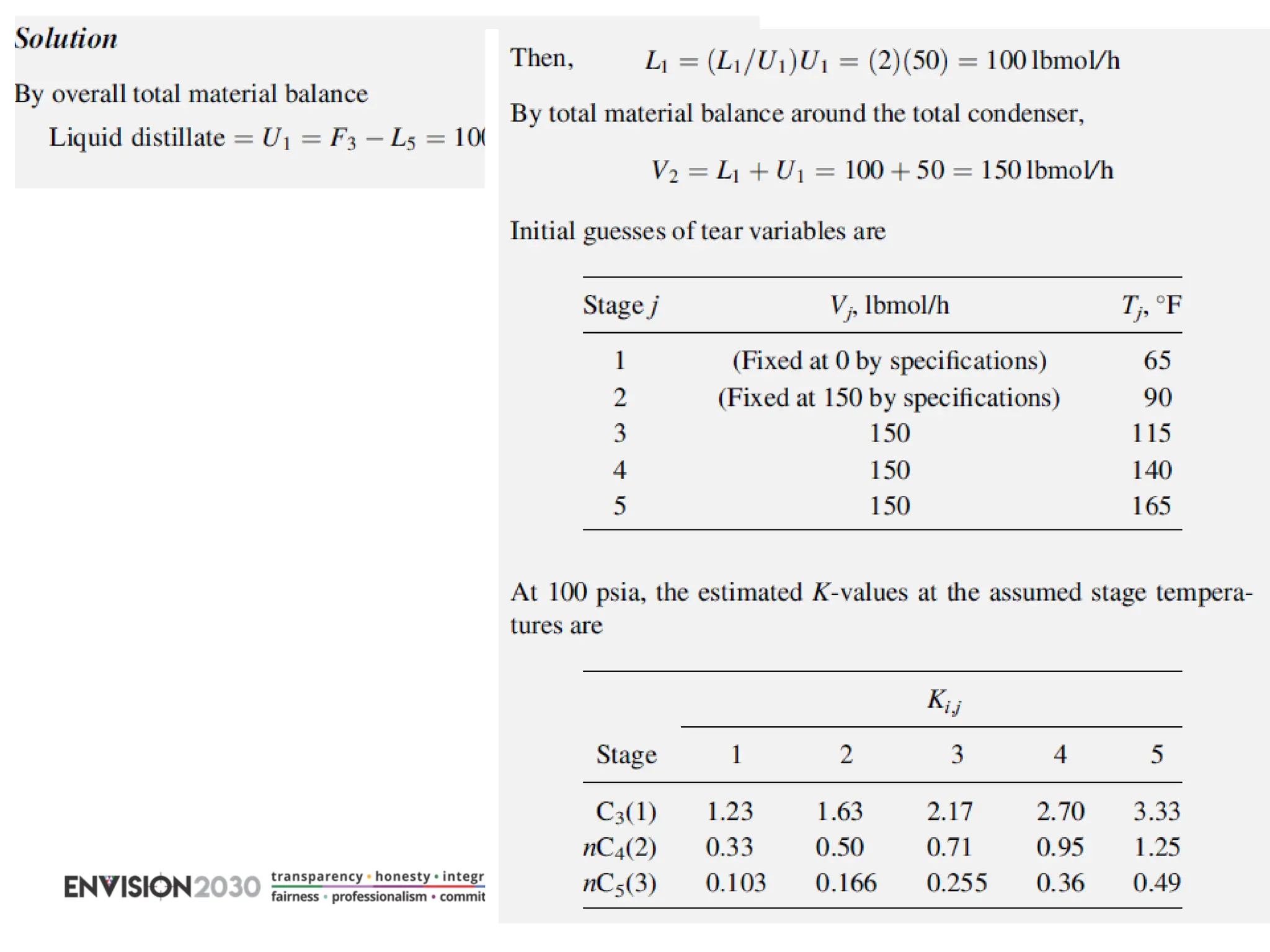
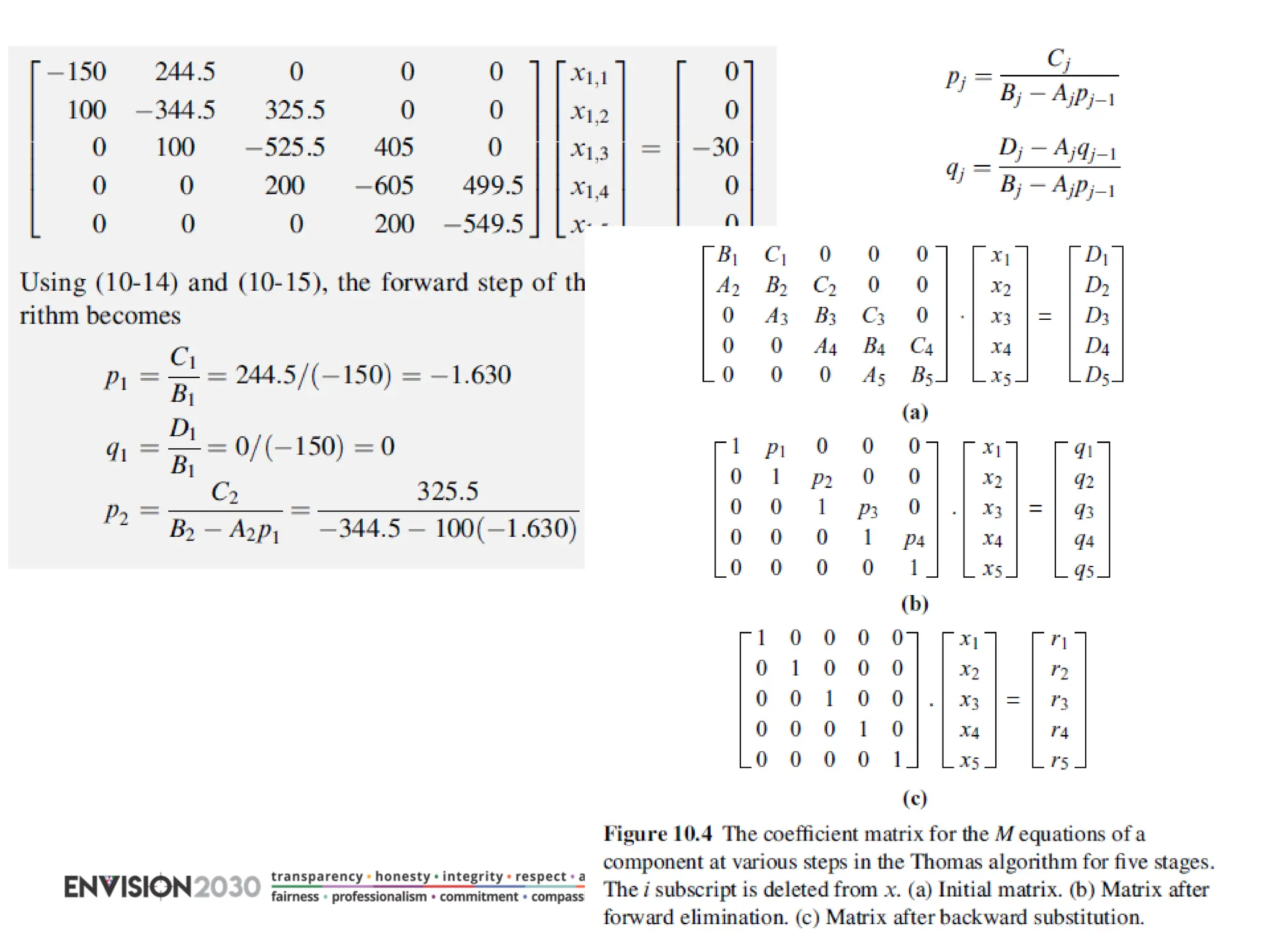
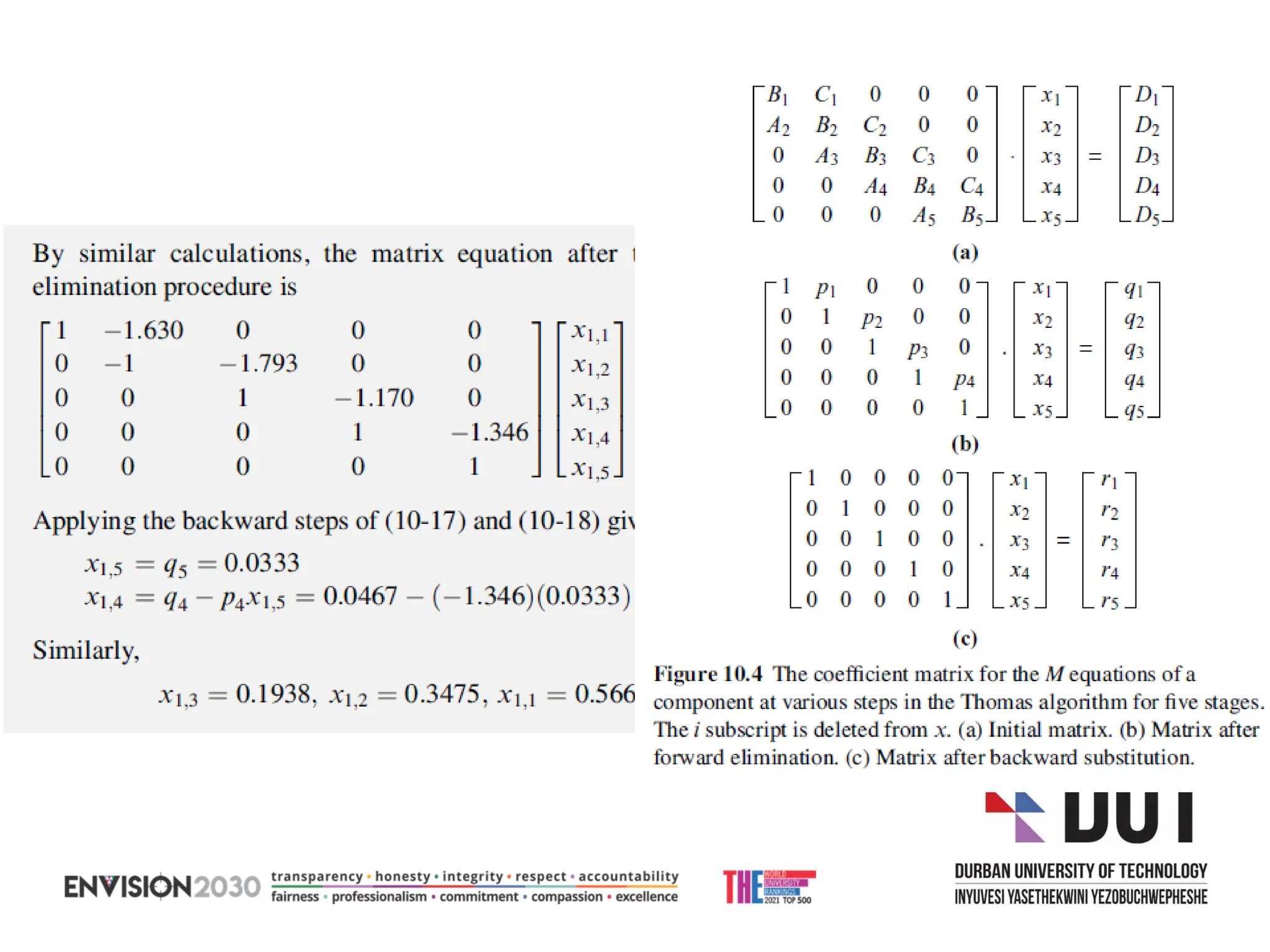
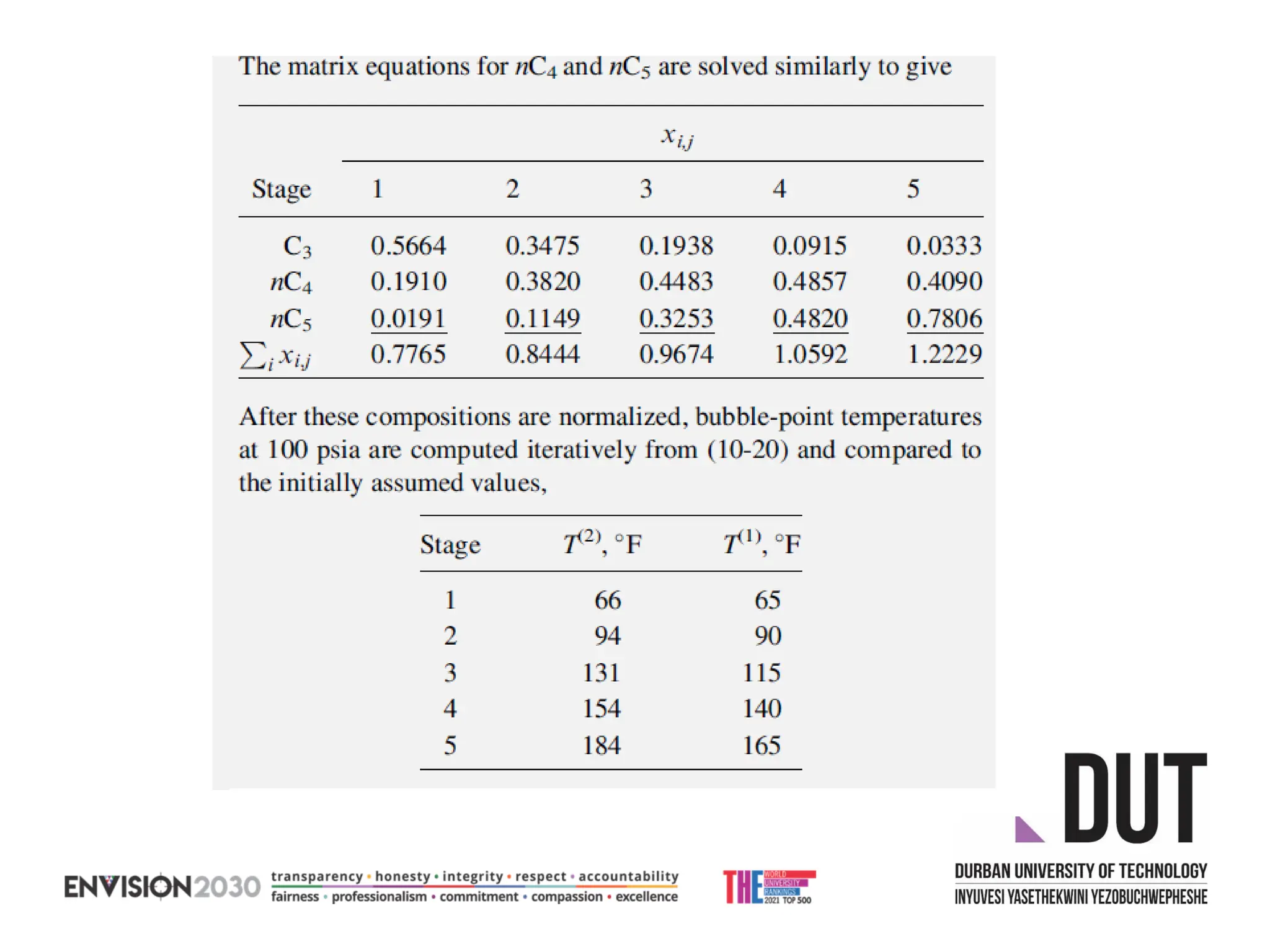
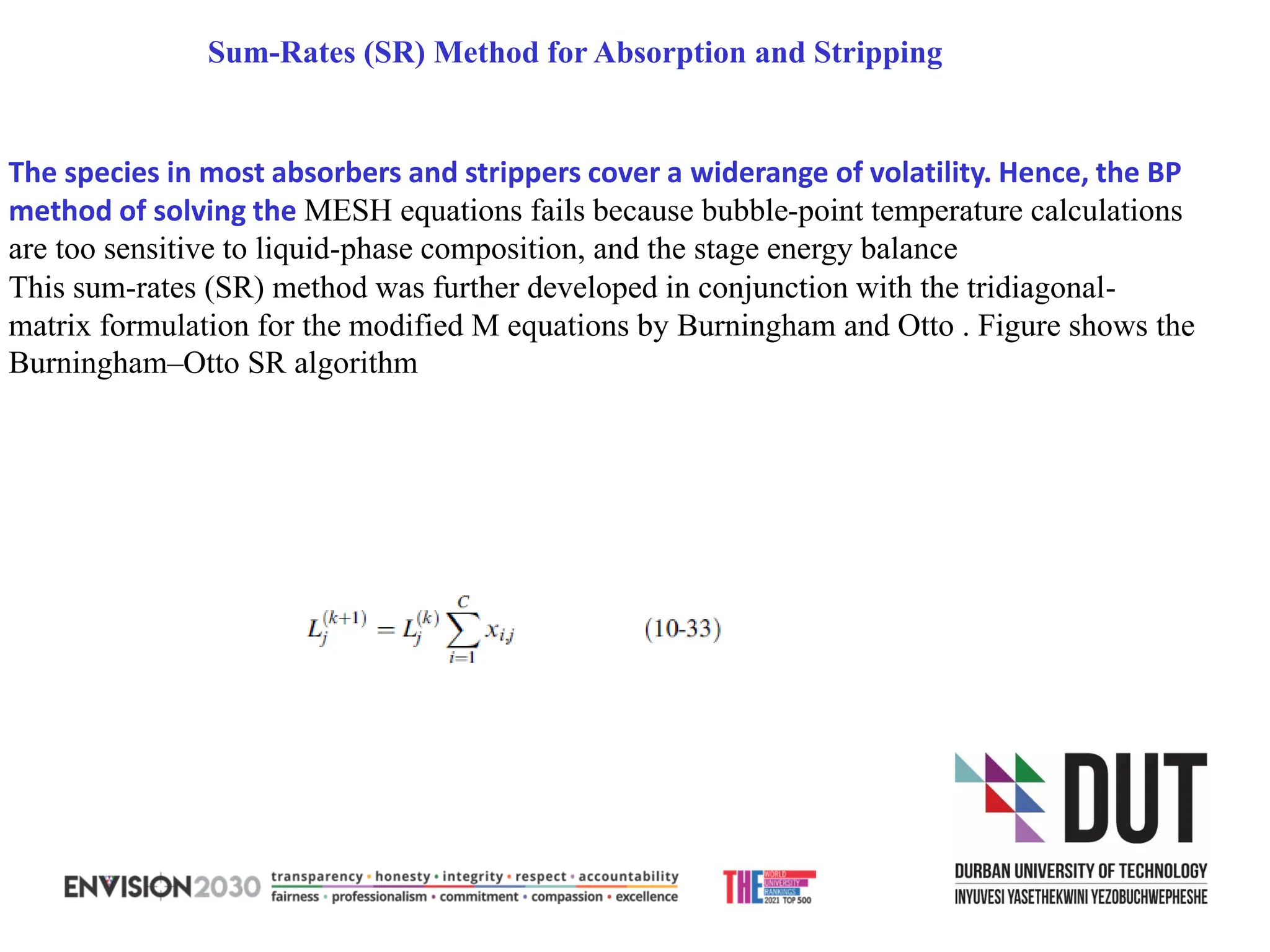
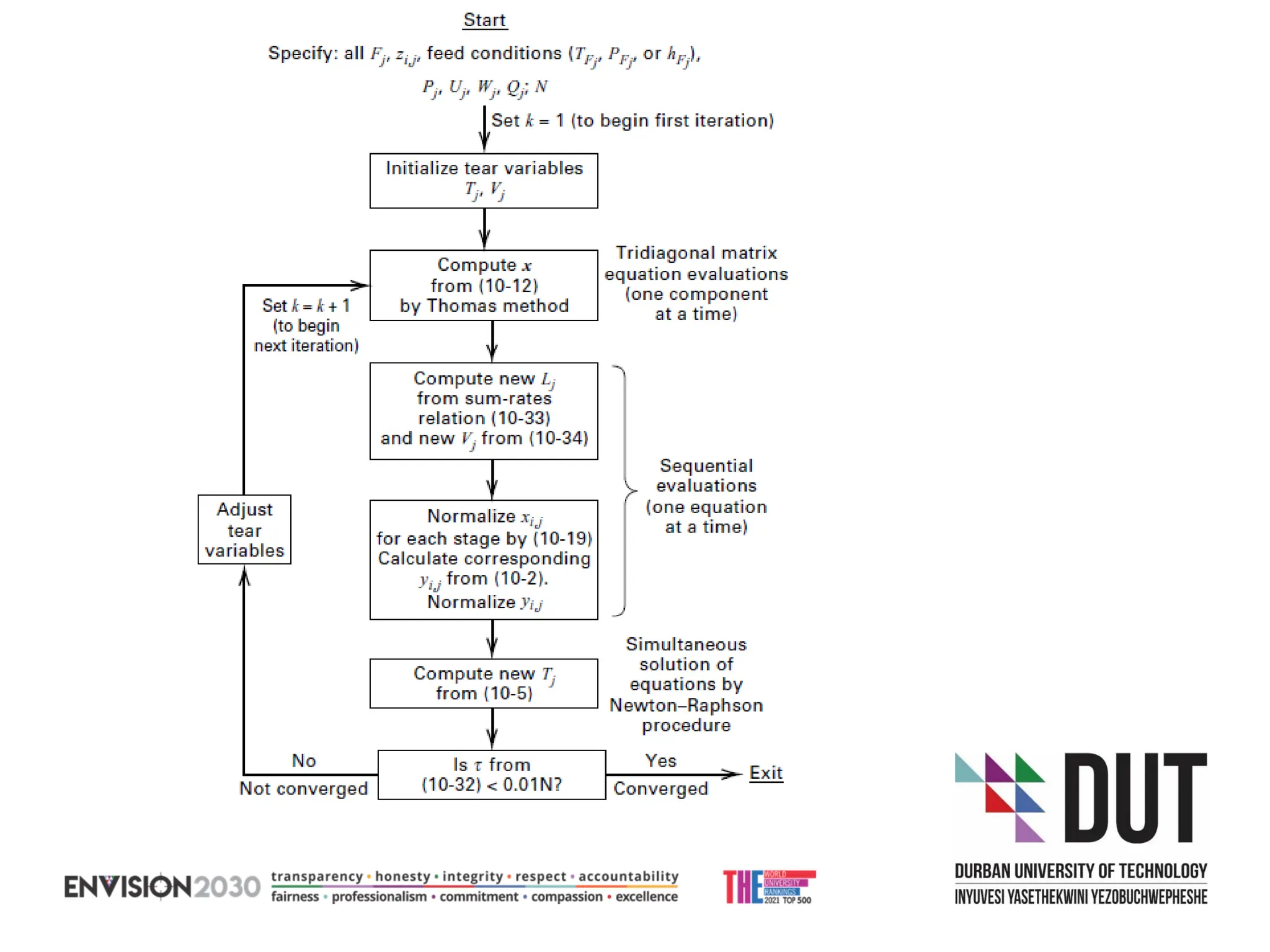
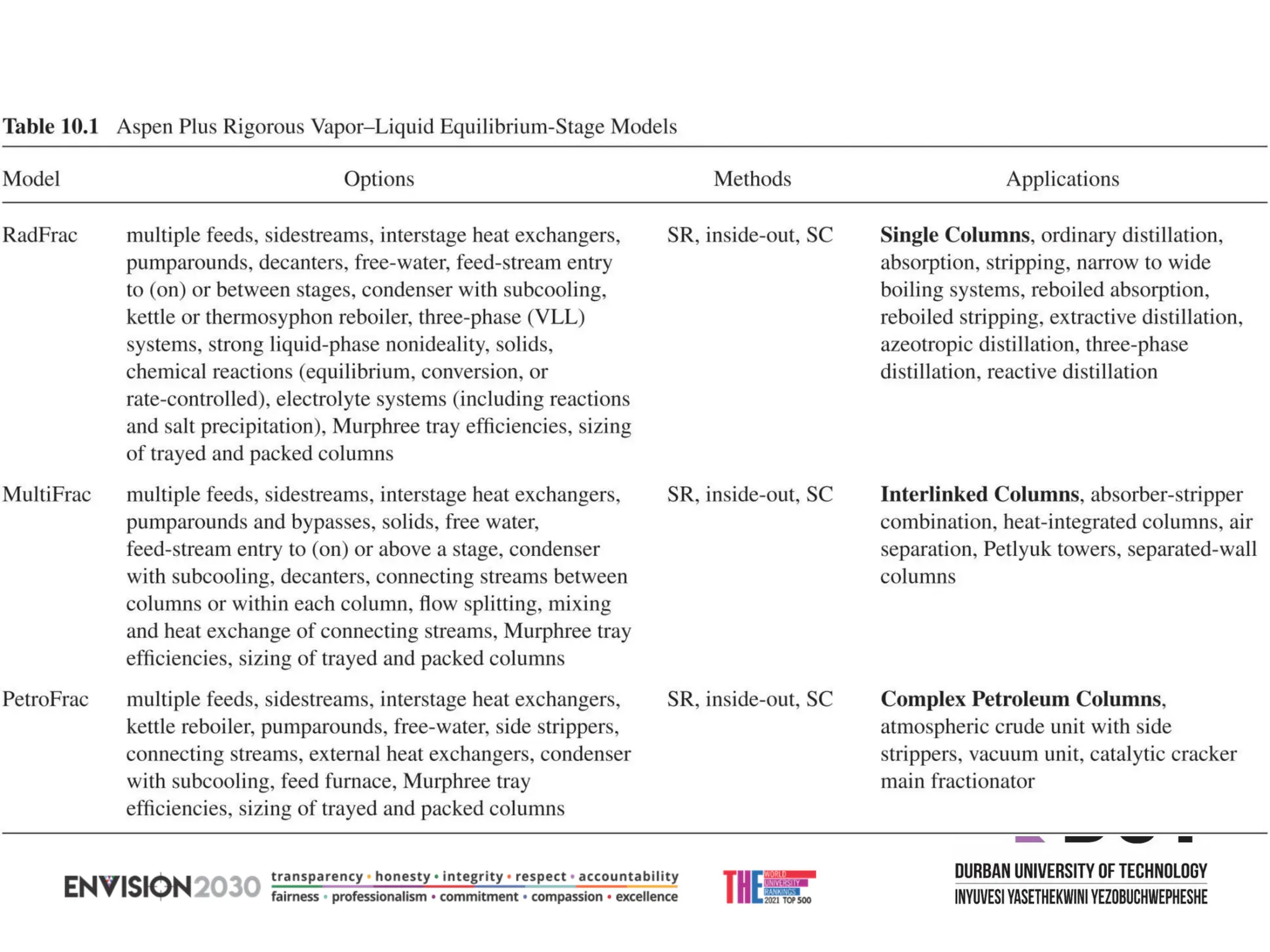
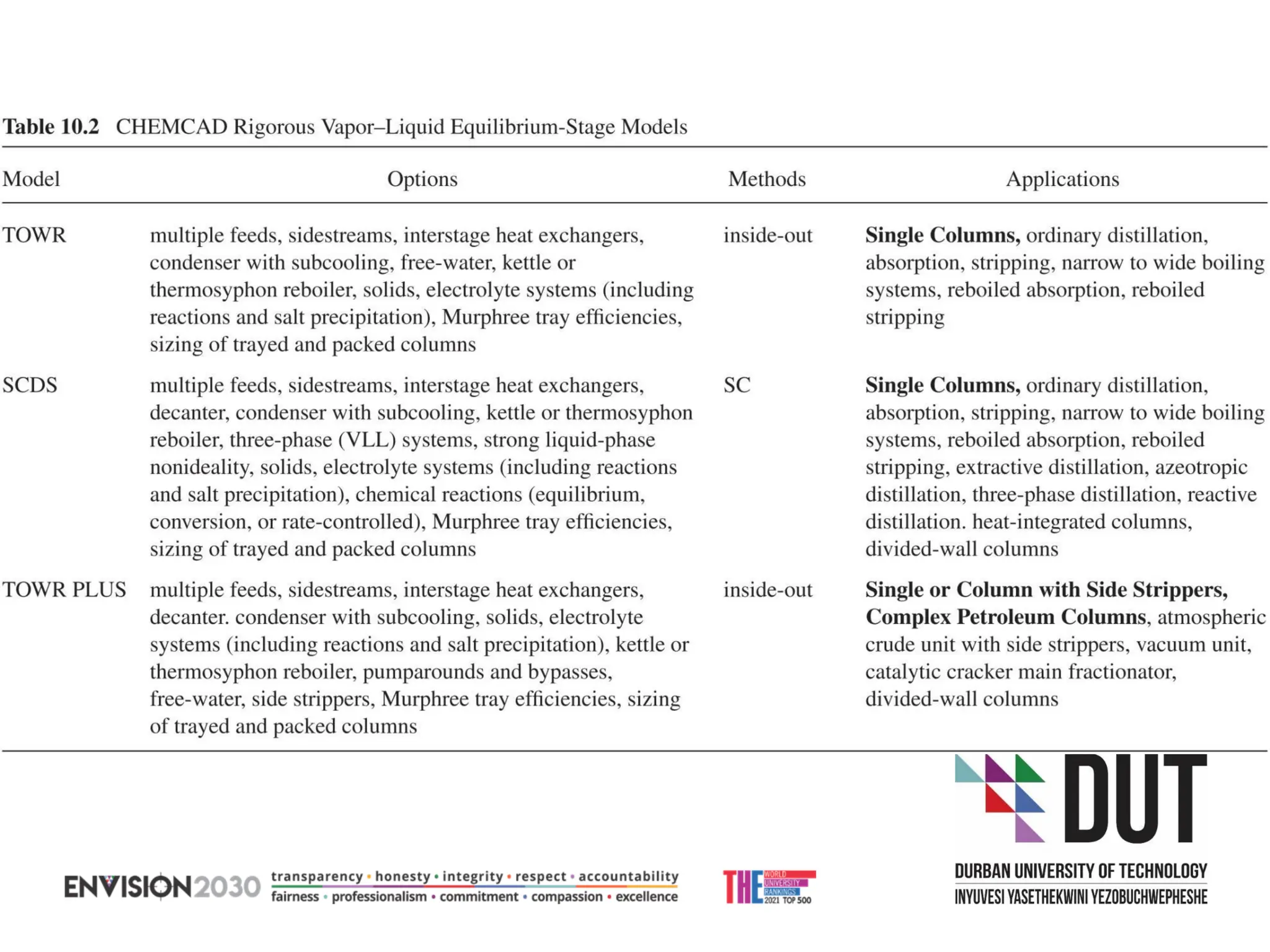
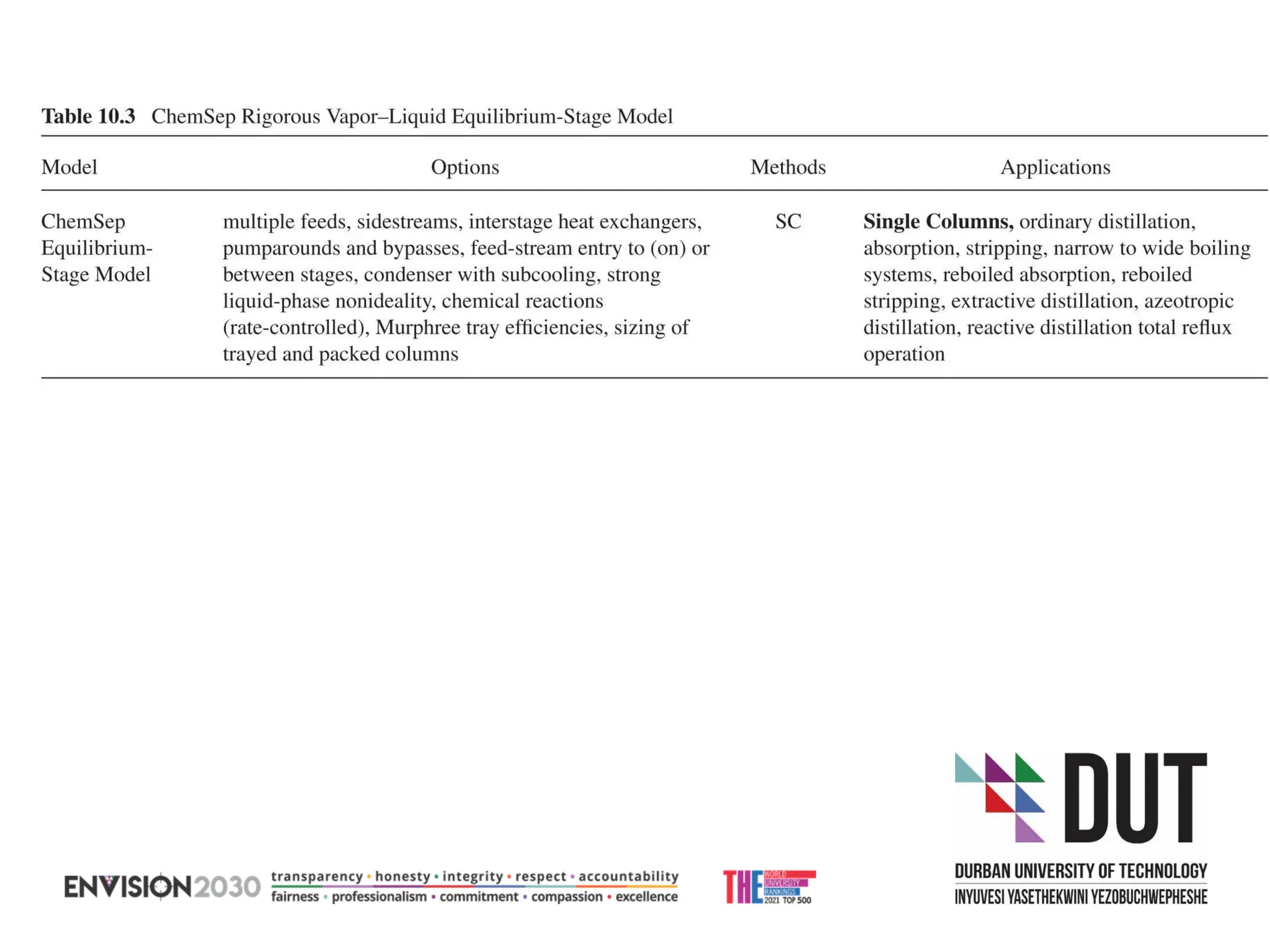
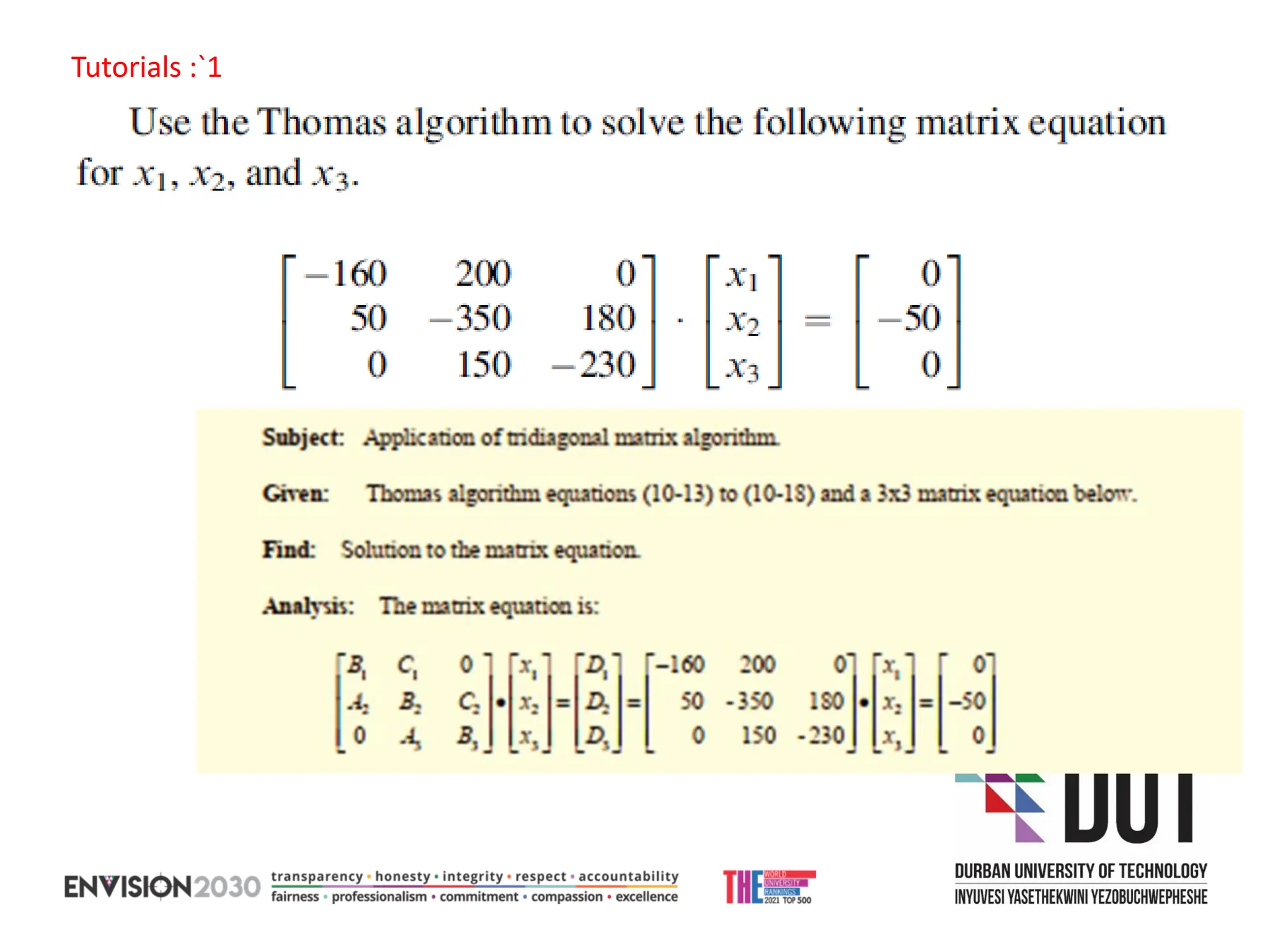
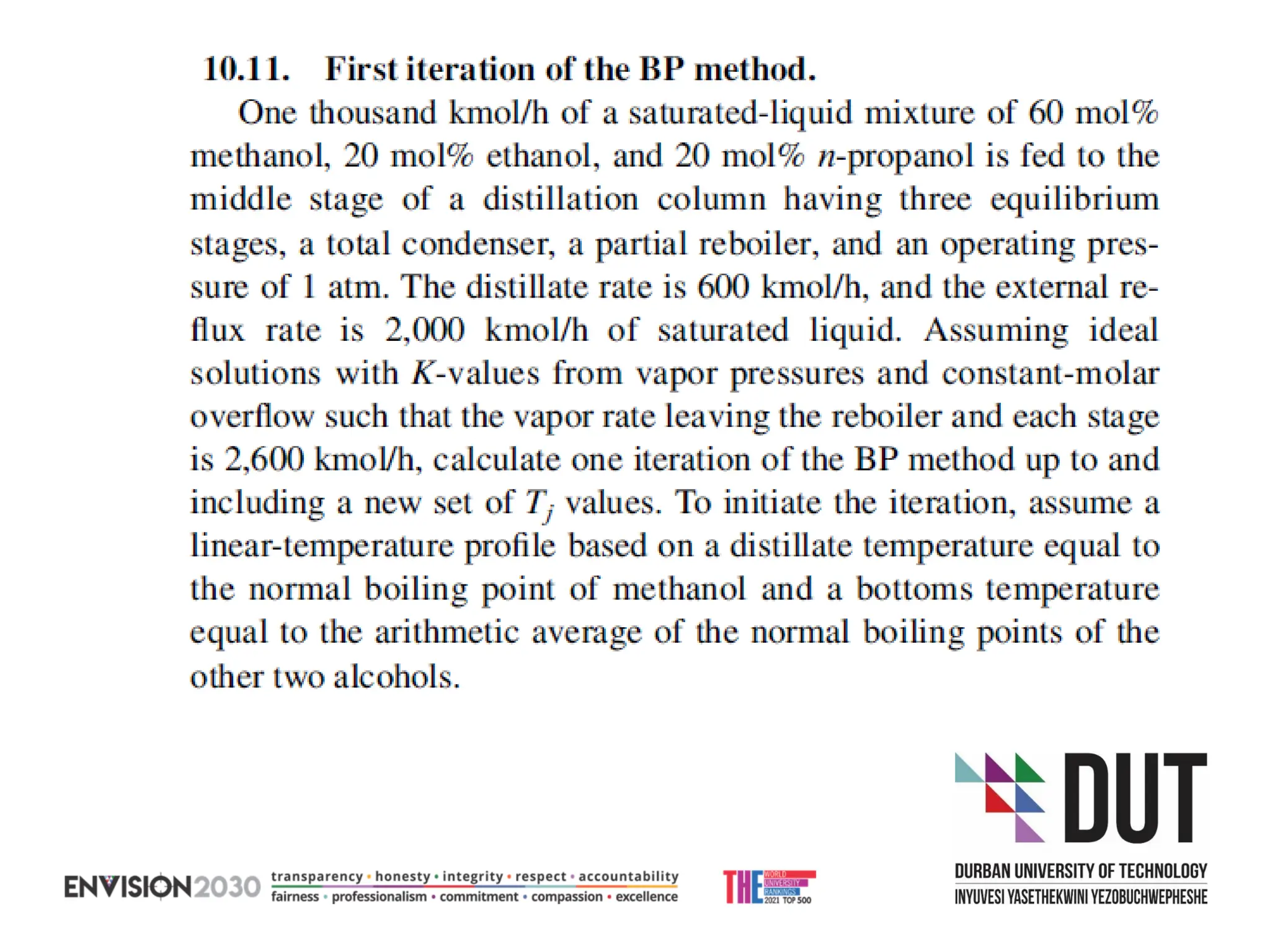
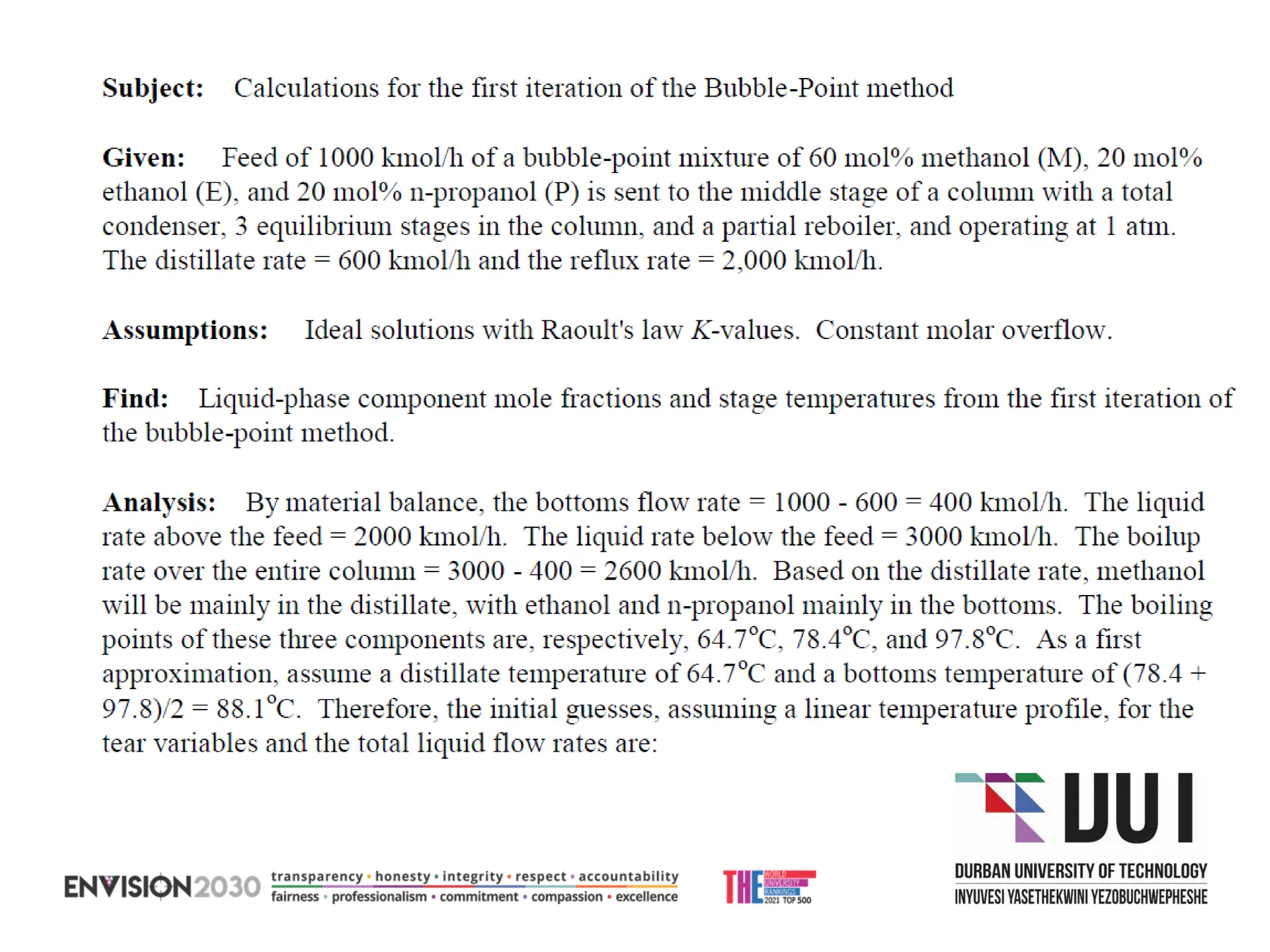
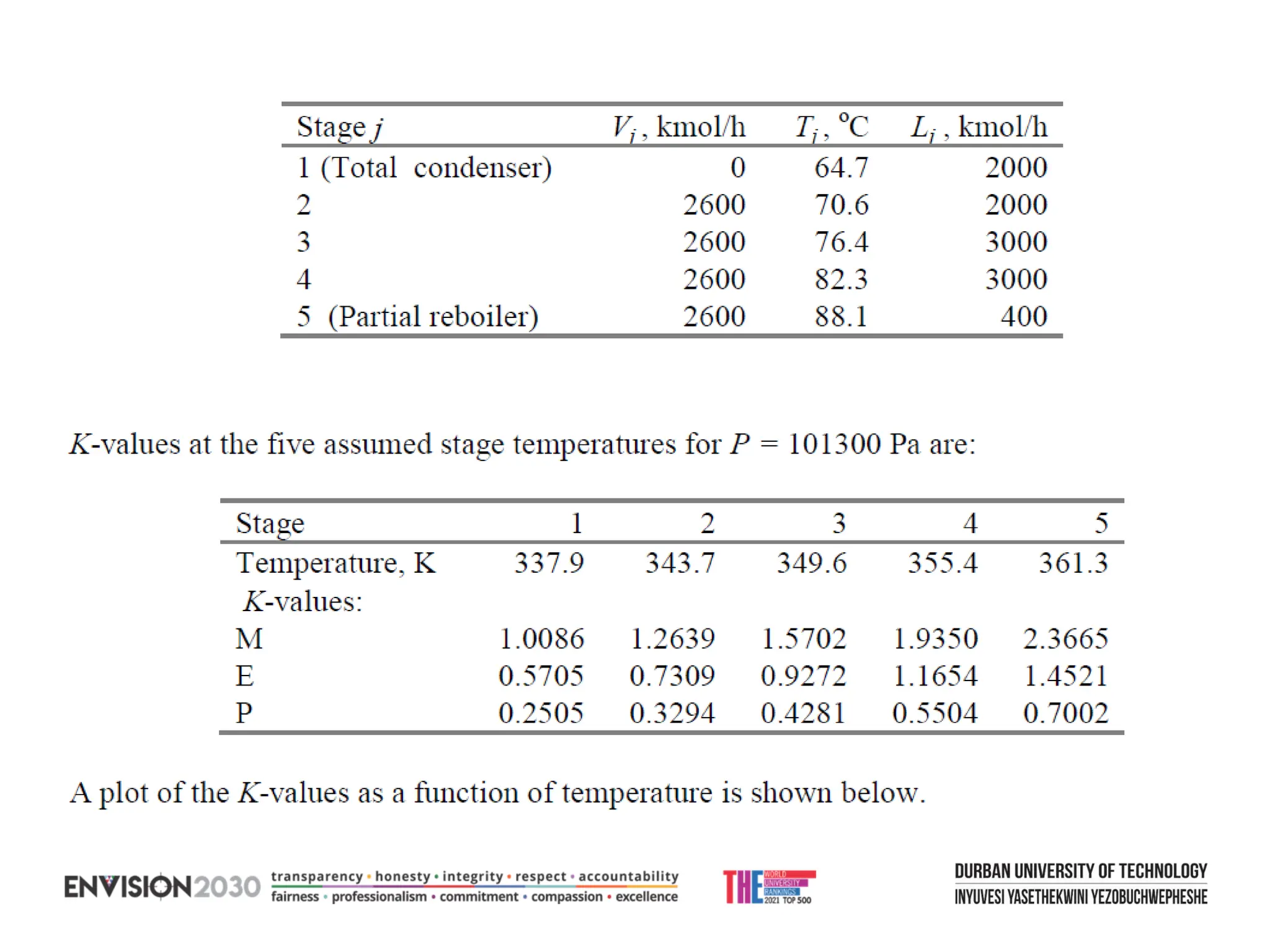
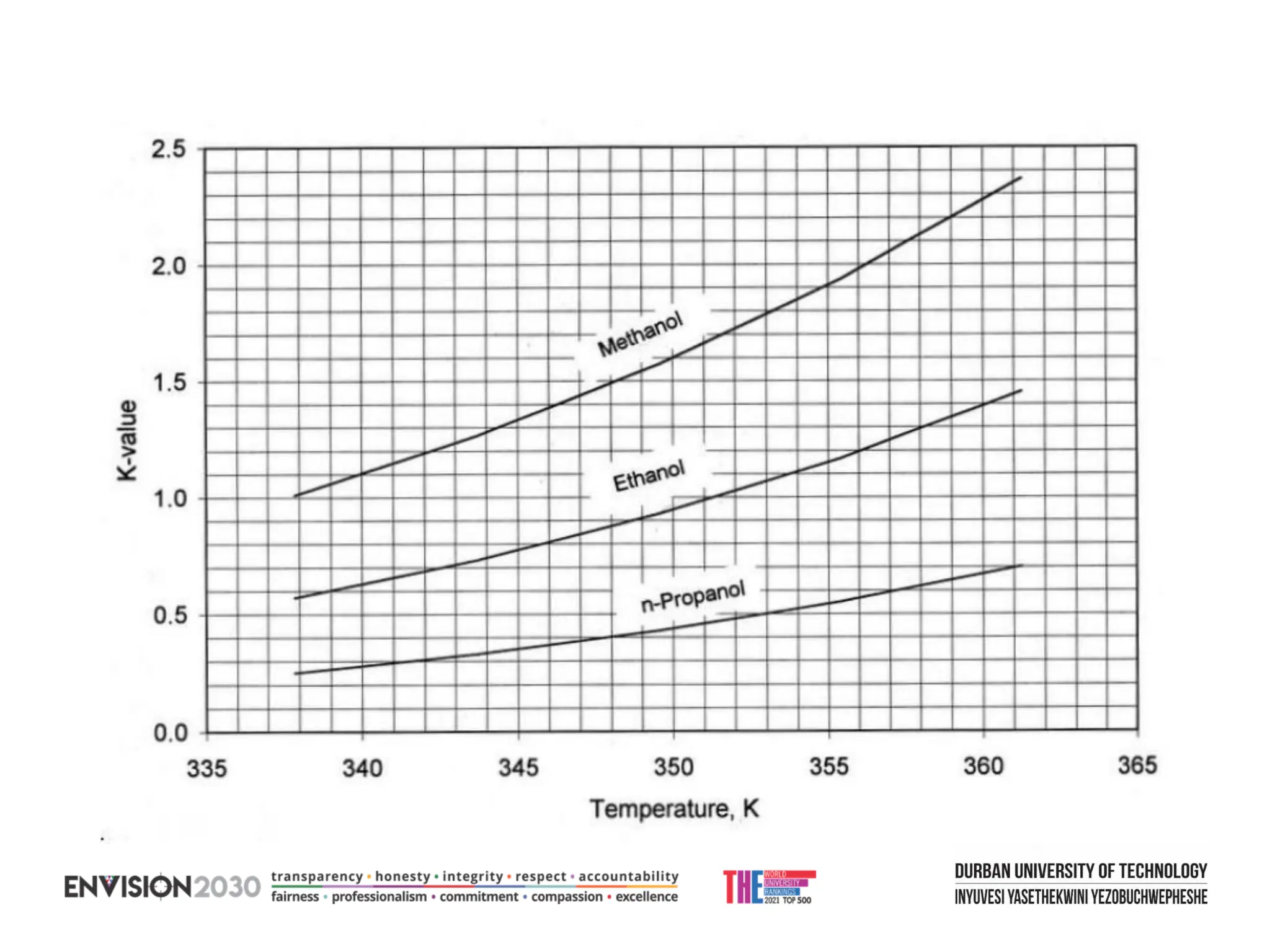
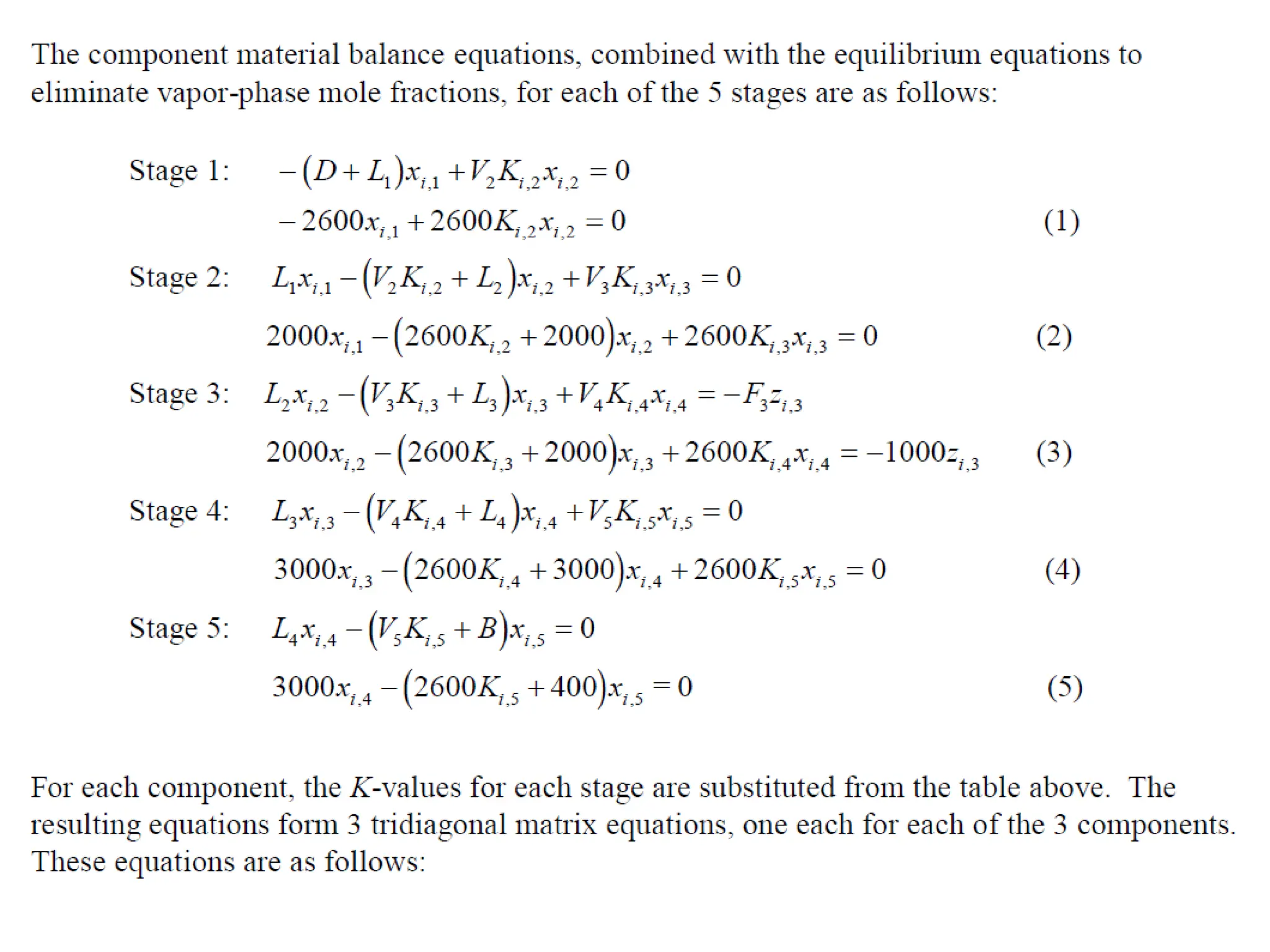
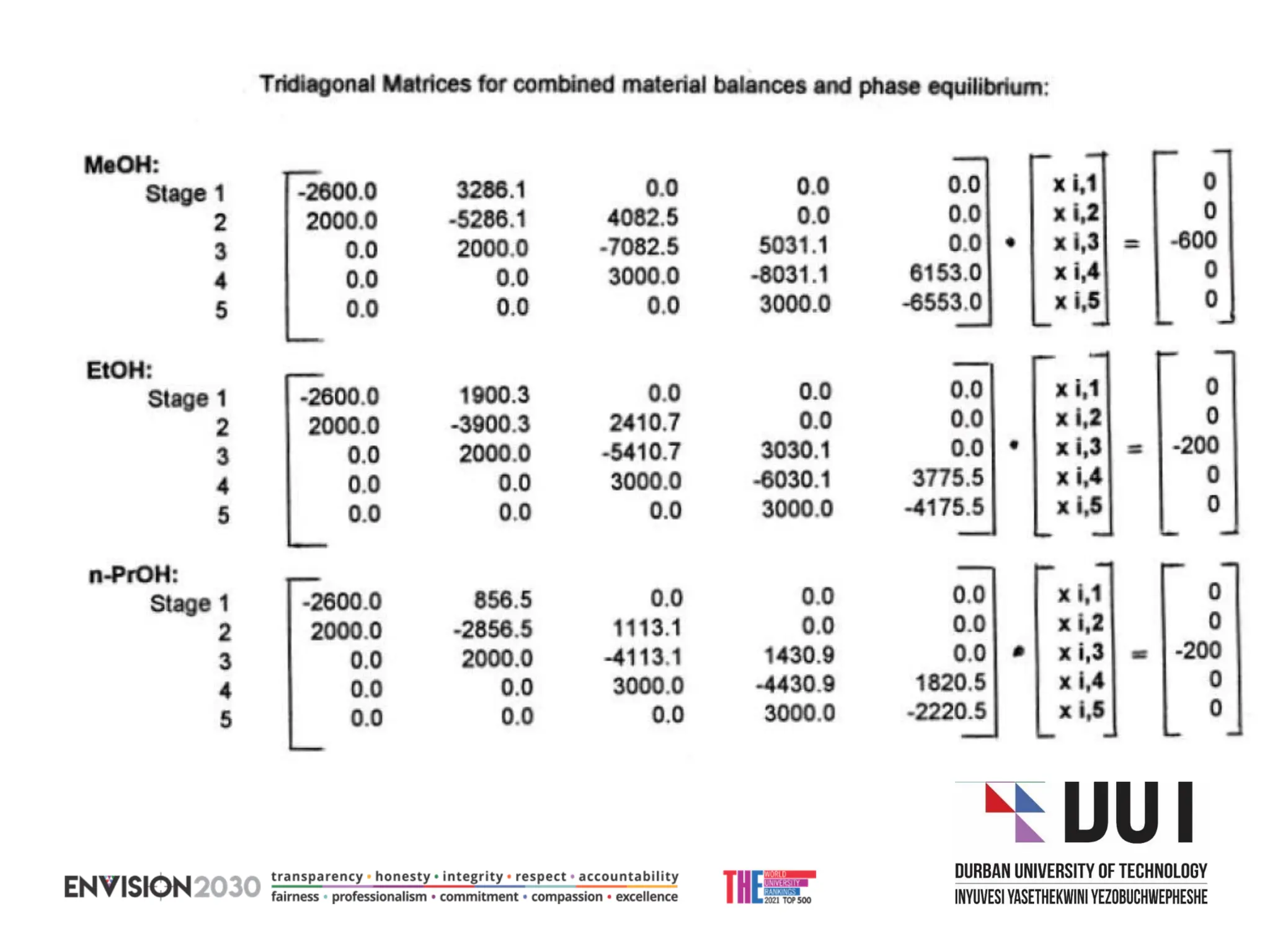
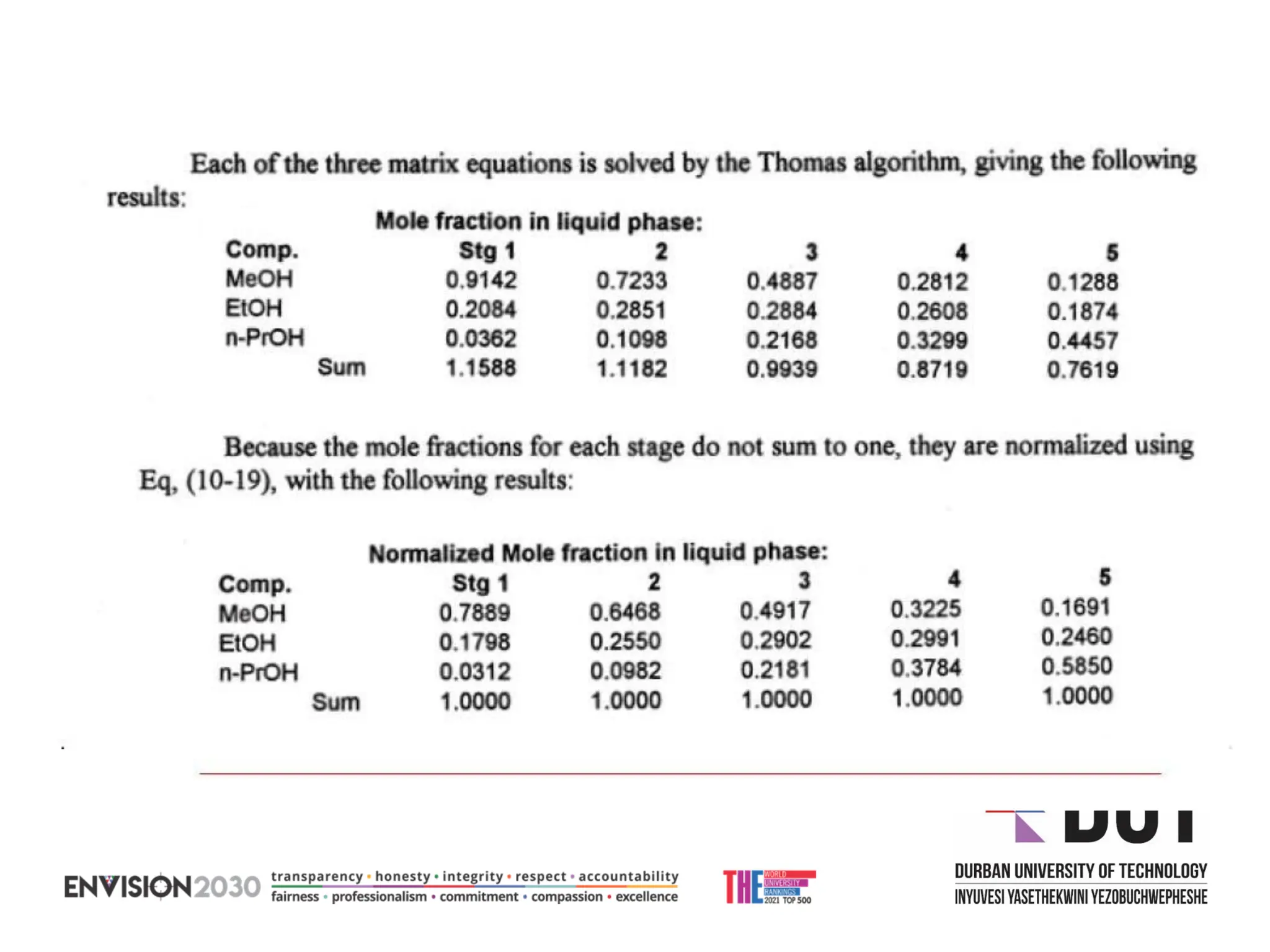
The document outlines lecture notes on separation technology, detailing the use of equilibrium-based methods for multicomponent separation processes like absorption, stripping, distillation, and extraction. It discusses mathematical modeling, solution procedures for nonlinear equations, and algorithms for calculating stage temperatures and compositions in process simulations. Additionally, the document contains information on assignments, test dates, and recommended textbooks for the course.