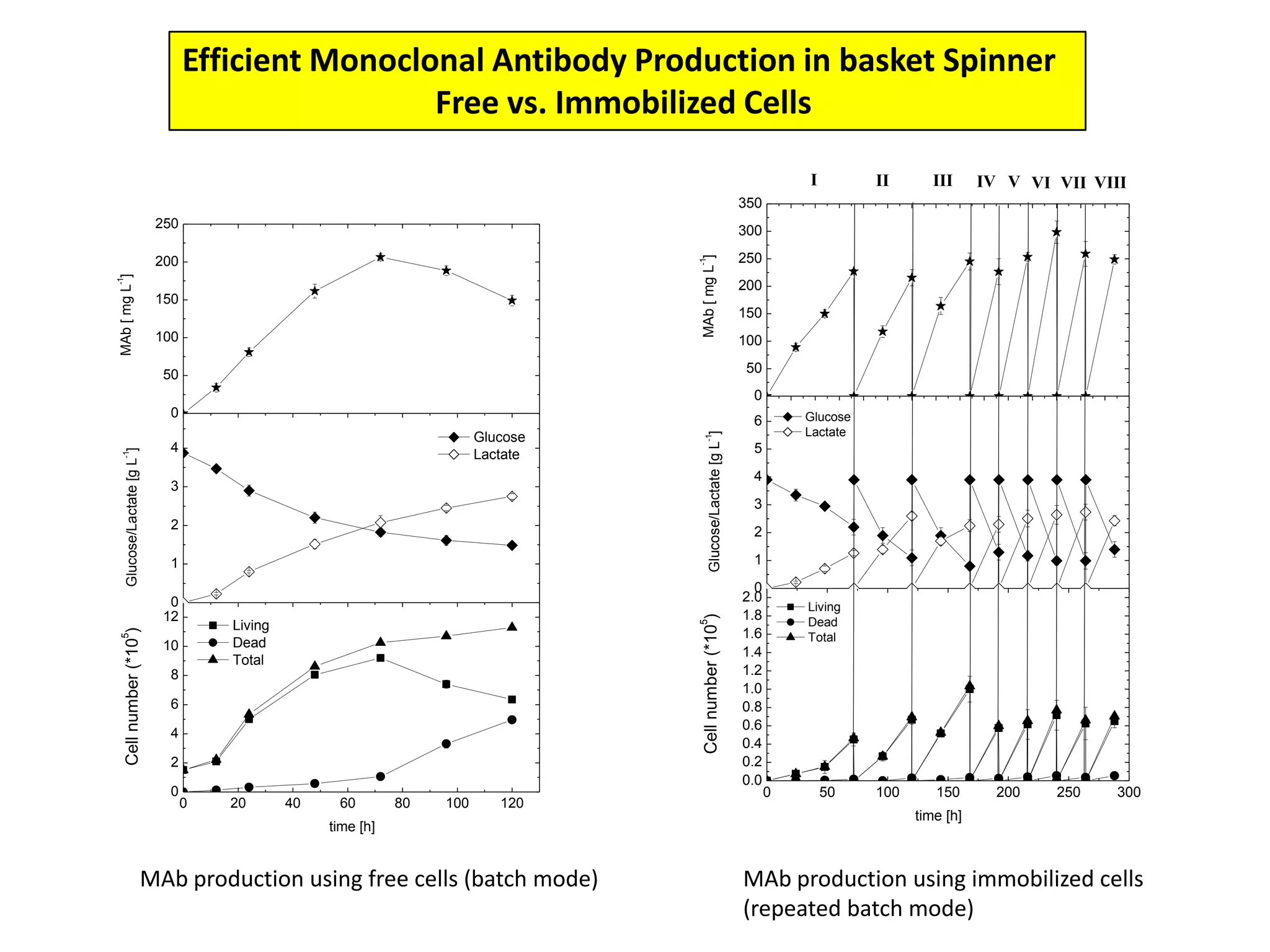
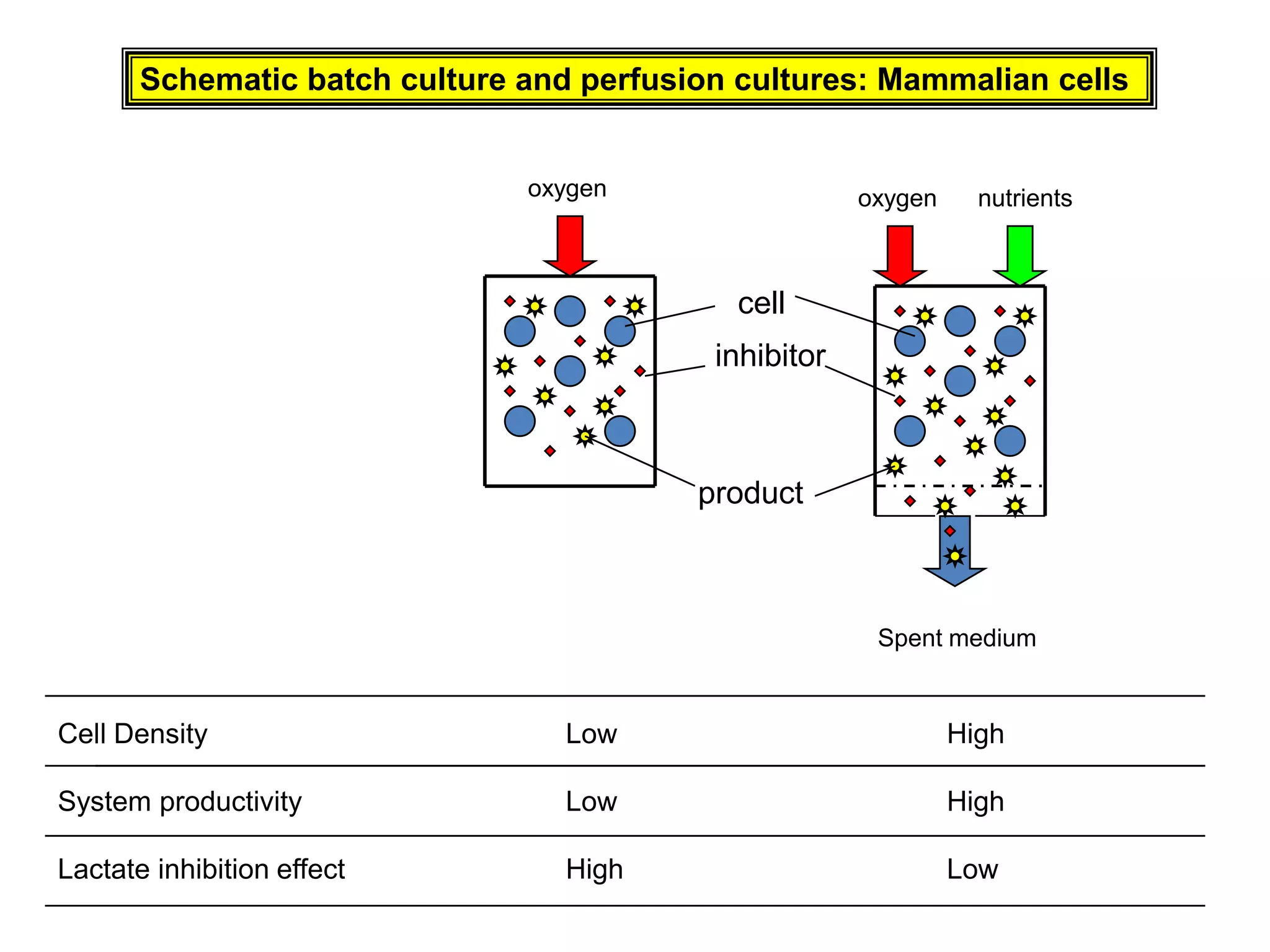
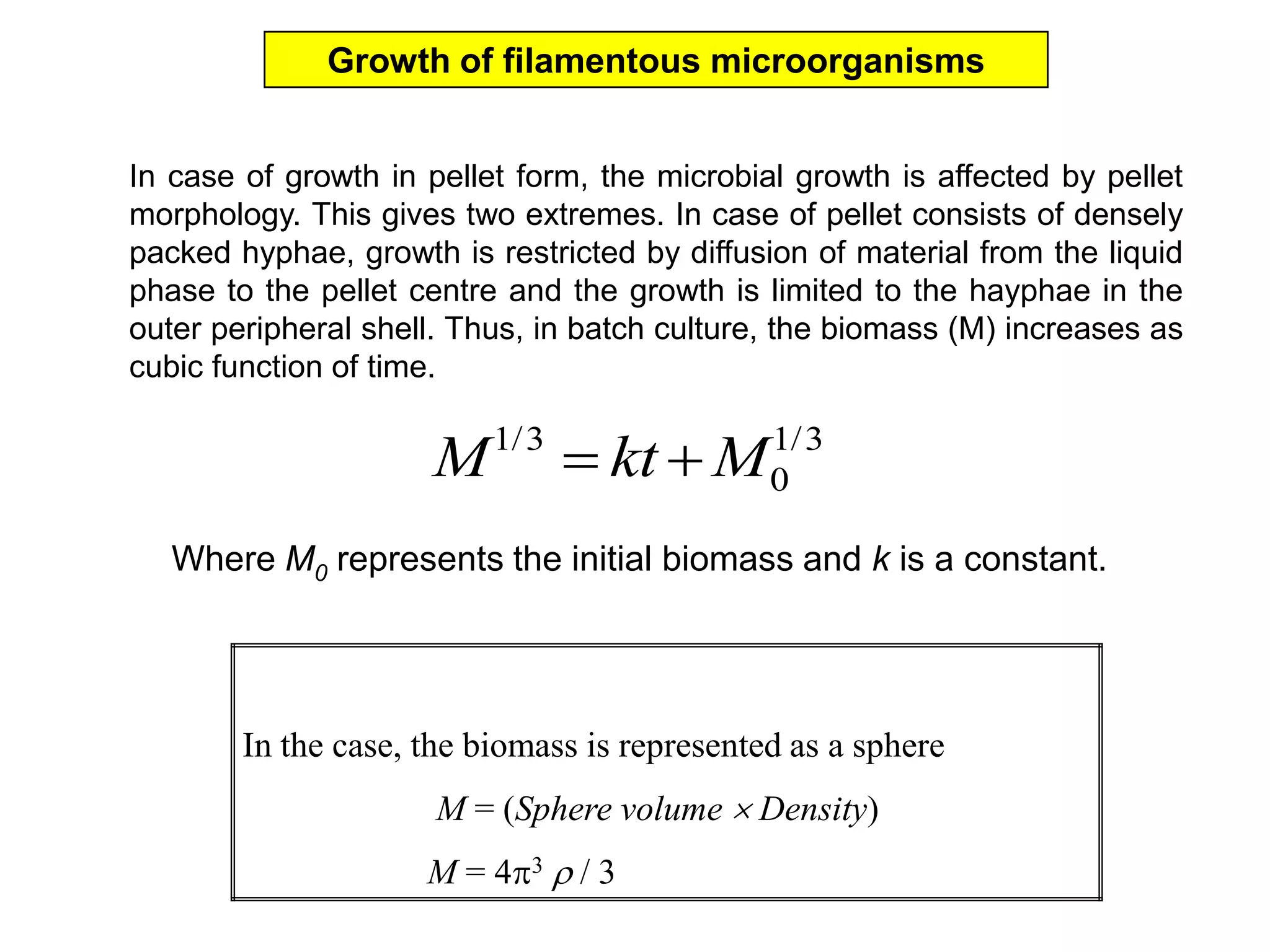
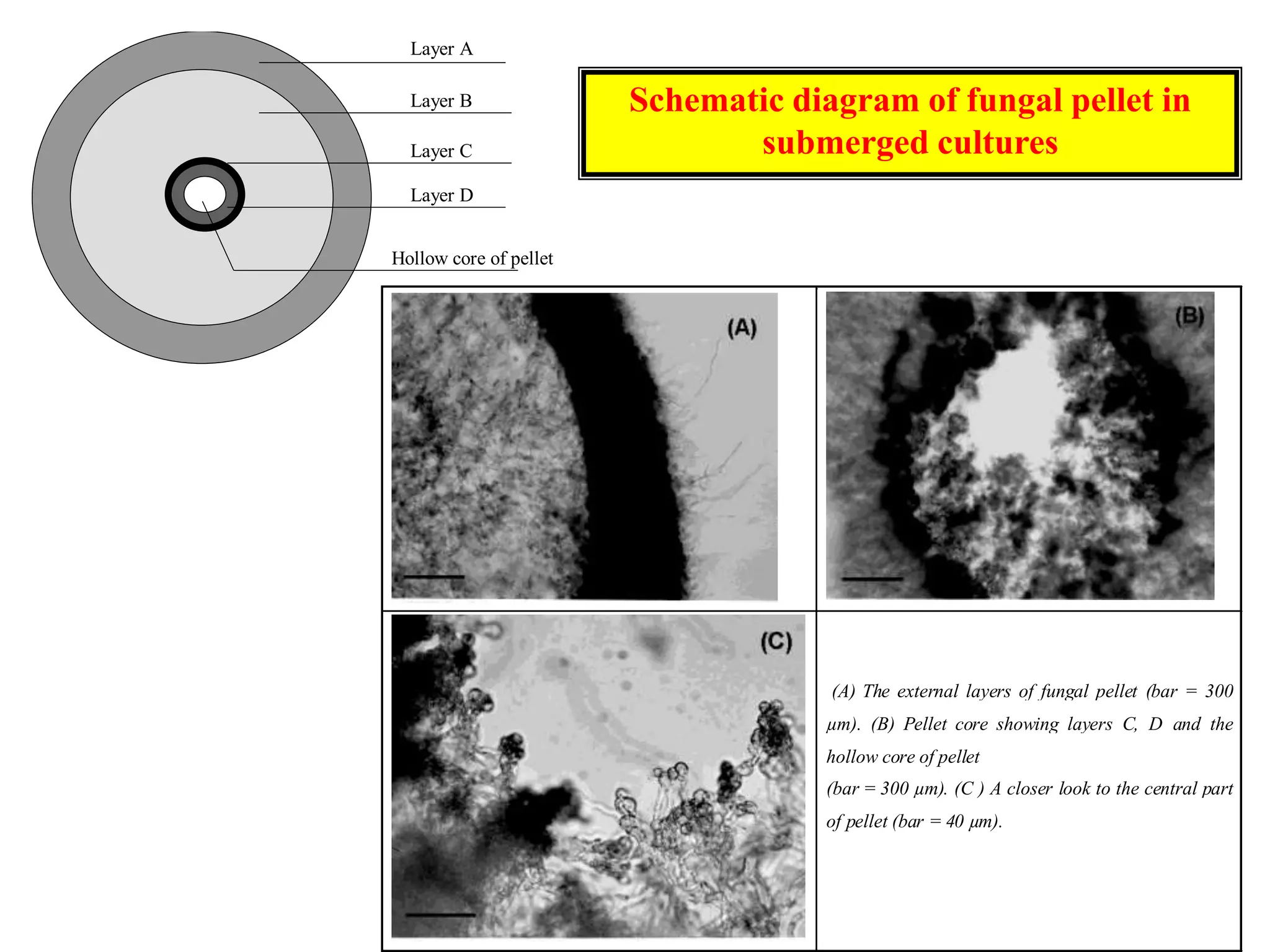
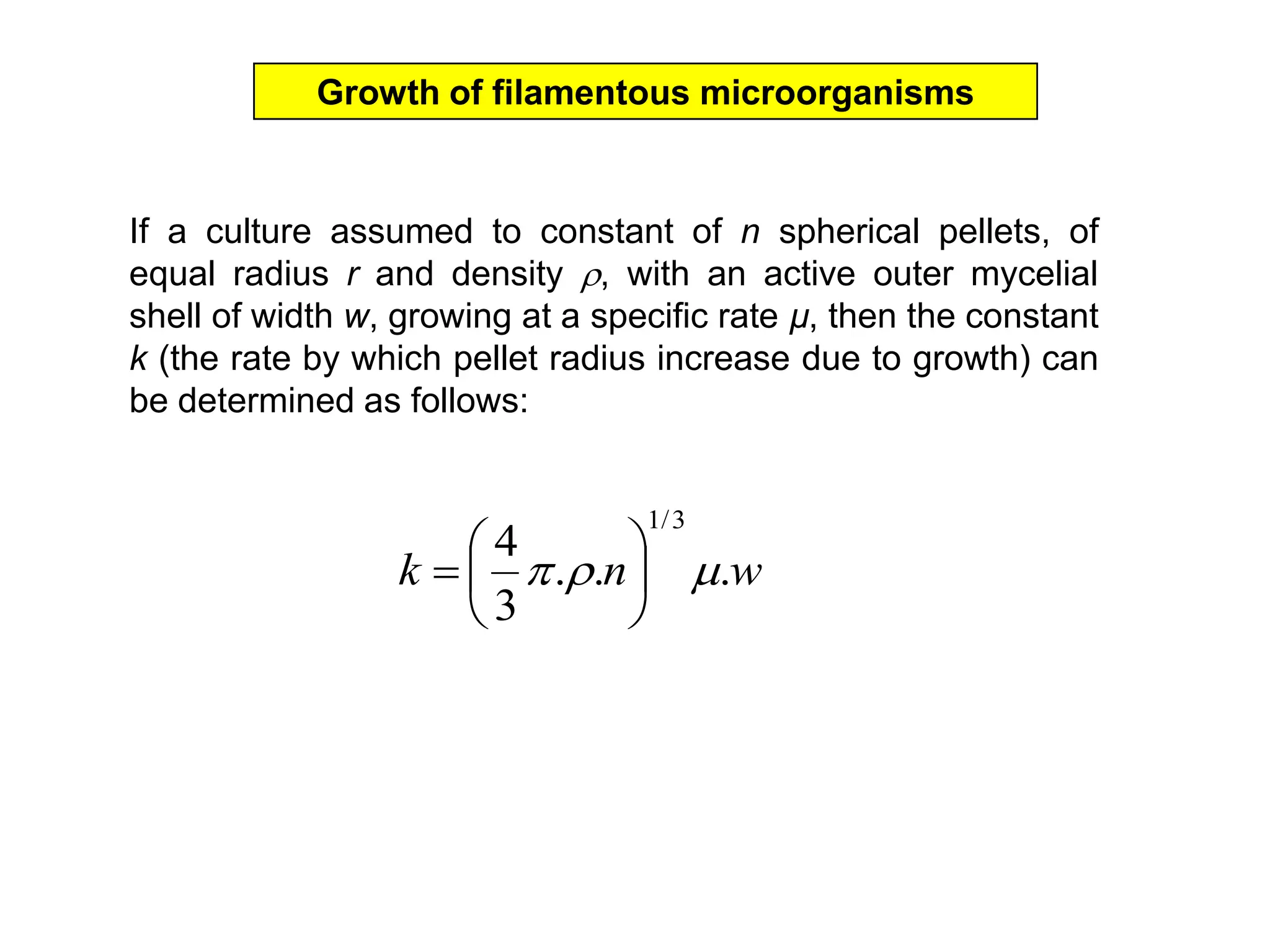
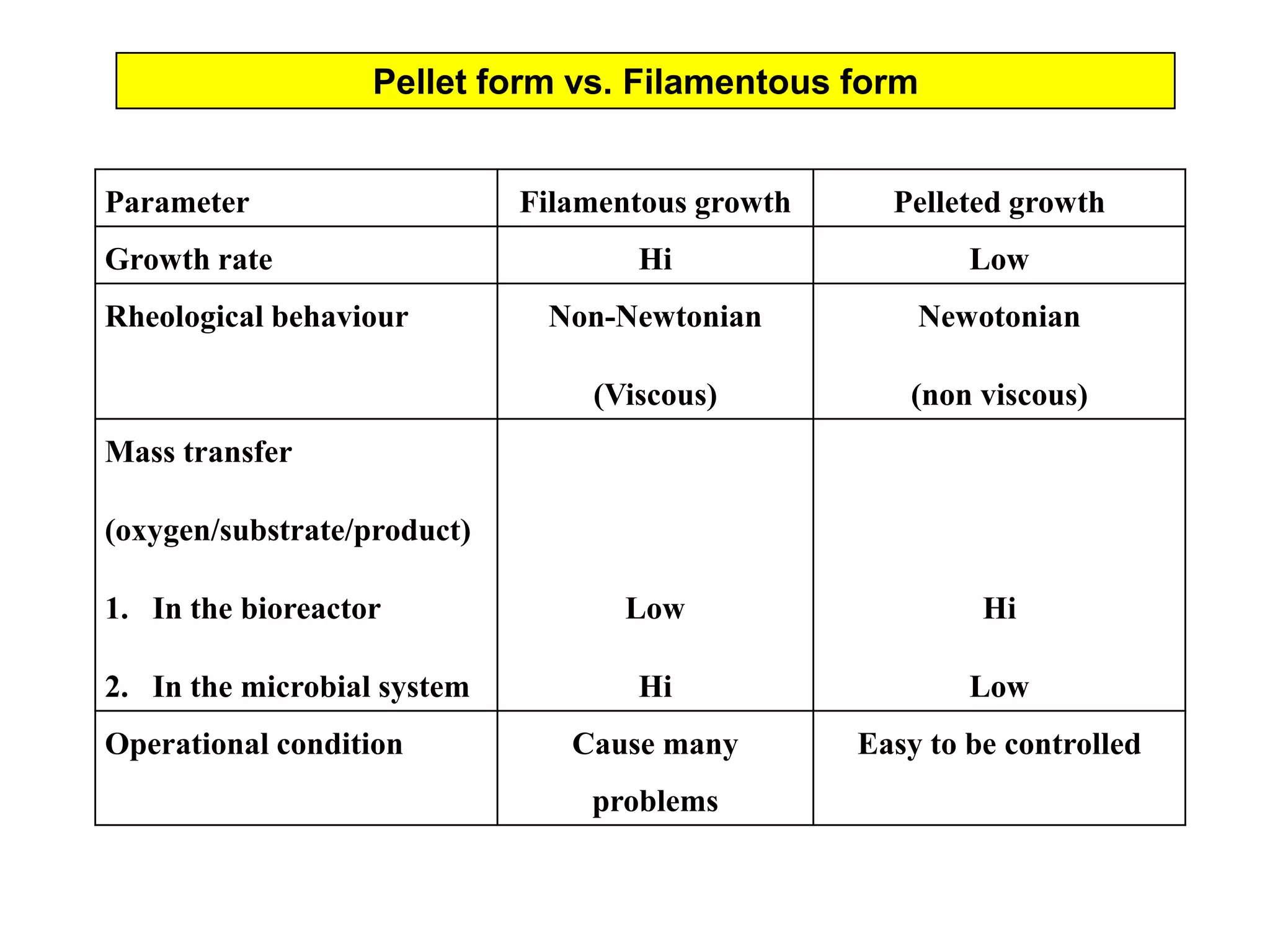
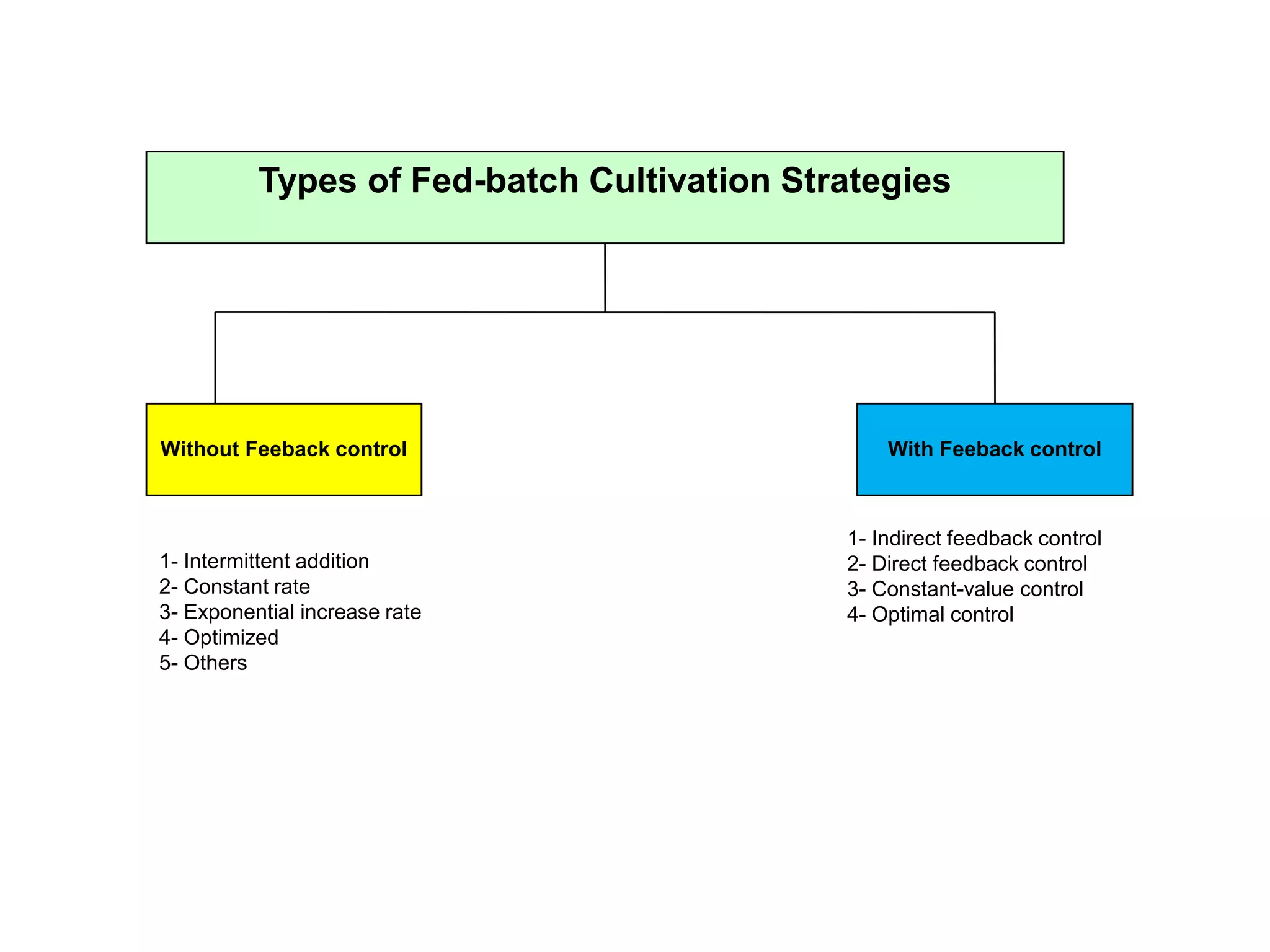
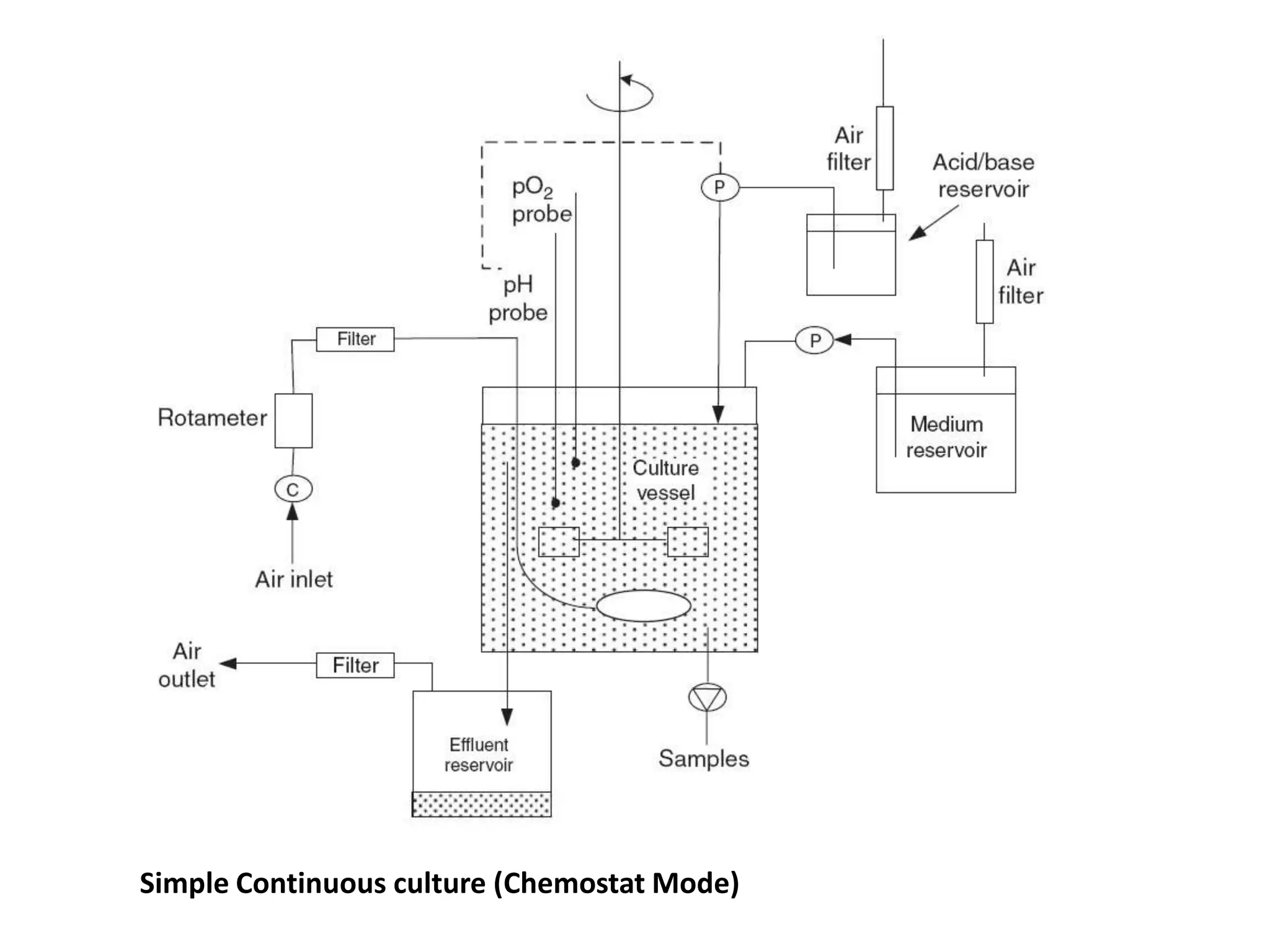
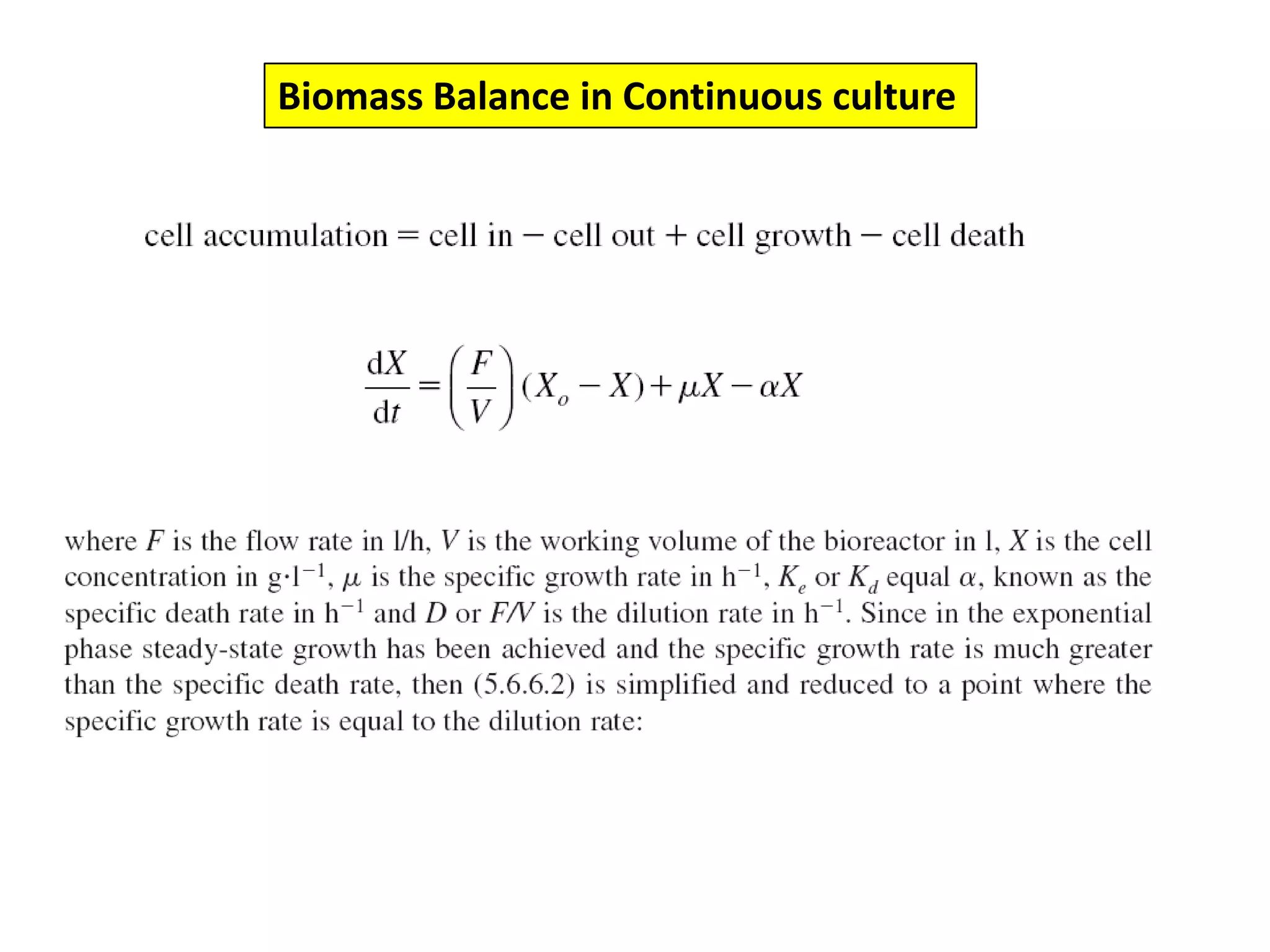
This document discusses different bioprocess cultivation systems and operation modes. It covers two-phase and three-phase cultivation systems, as well as free and immobilized cell systems. Batch, fed-batch, and continuous cultivation modes are described in detail. Specific topics covered include microbial growth curves, factors affecting lag phase, kinetics of exponential and stationary phases, and product formation under different operation modes. Advantages of fed-batch cultivation like avoiding inhibition and catabolite repression are highlighted. High cell density cultivation using exponential feeding strategies is also summarized.

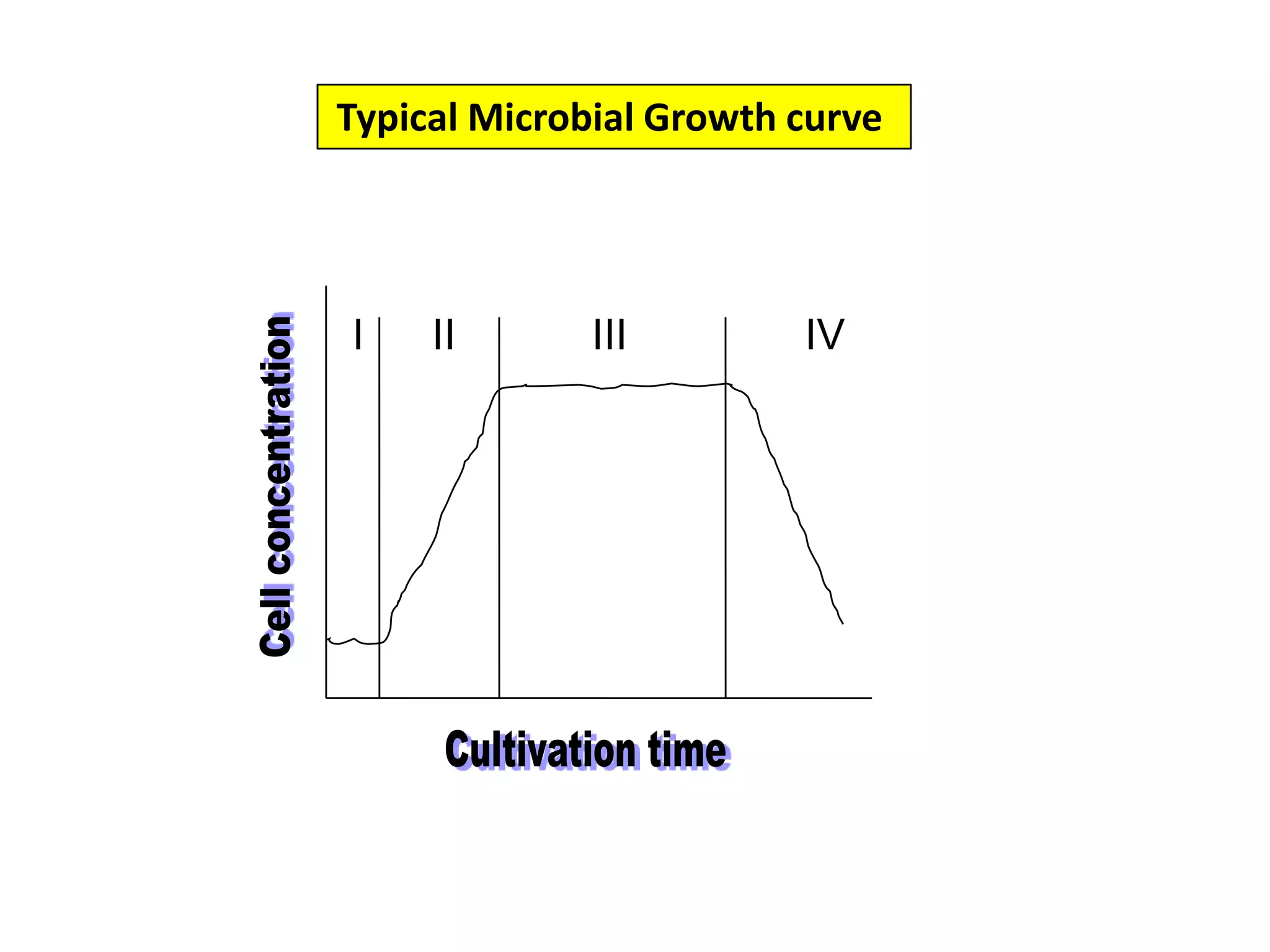
![Typical Microbial Growth curve II- The Log Phase:During this phase, cells grow exponentially with time. The relation between time and cell growth during this phase can be described simply as follows:Where, X, is the concentration of microbial biomass, t, is time in hours and µ, is the specific growth rate in [h-1].In general, it is easy to visualize the exponential growth of unicellular organisms which replicate by binary fission. Also, animal and plant cells in suspension culture behave very similar to unicellular microorganisms.](https://image.slidesharecdn.com/lecture5-bioprocesstechnologyoperationmodeandscale-100716233917-phpapp02/75/Lecture-5-bioprocess-technology-operation-mode-and-scale-8-2048.jpg)
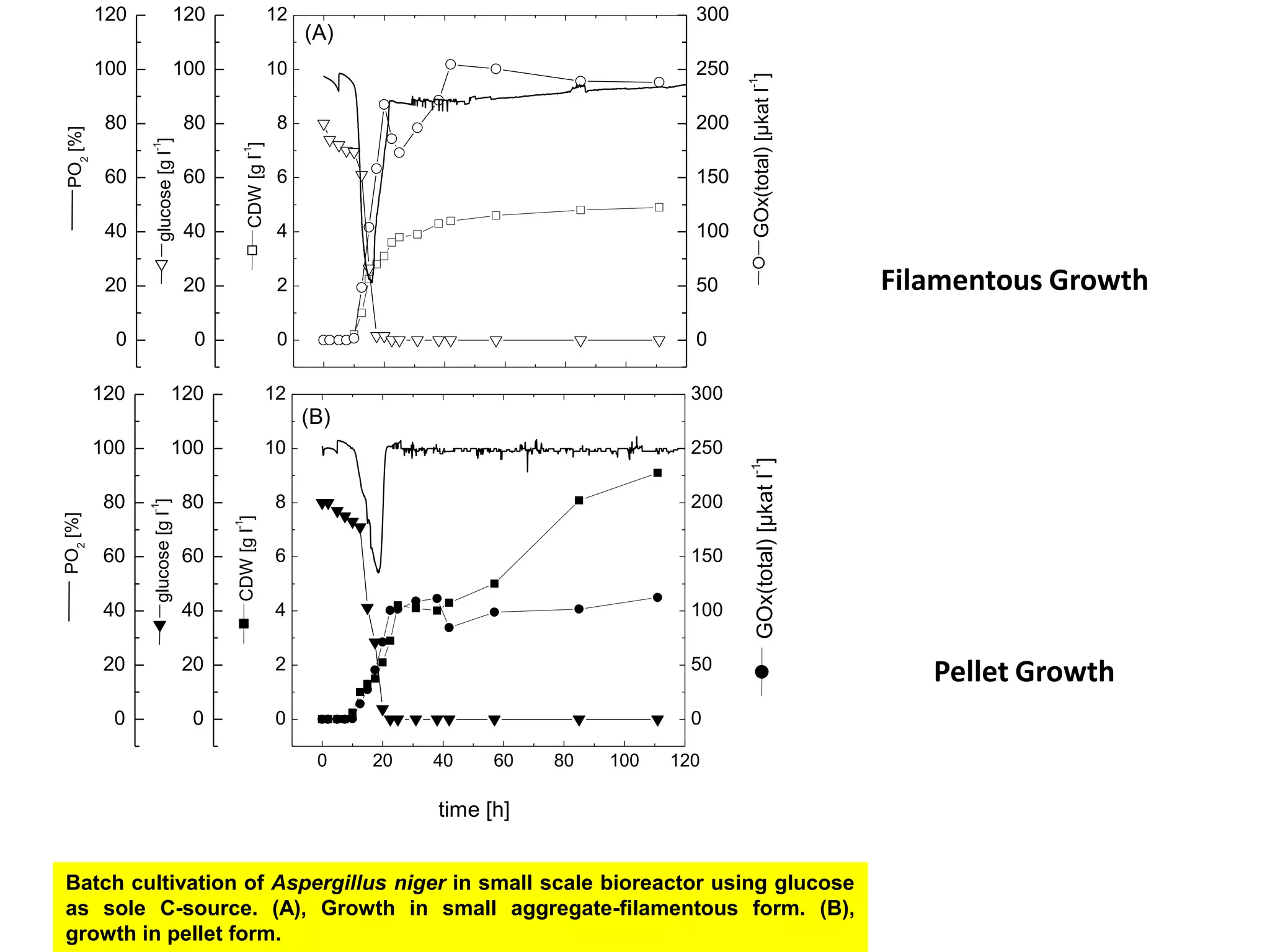
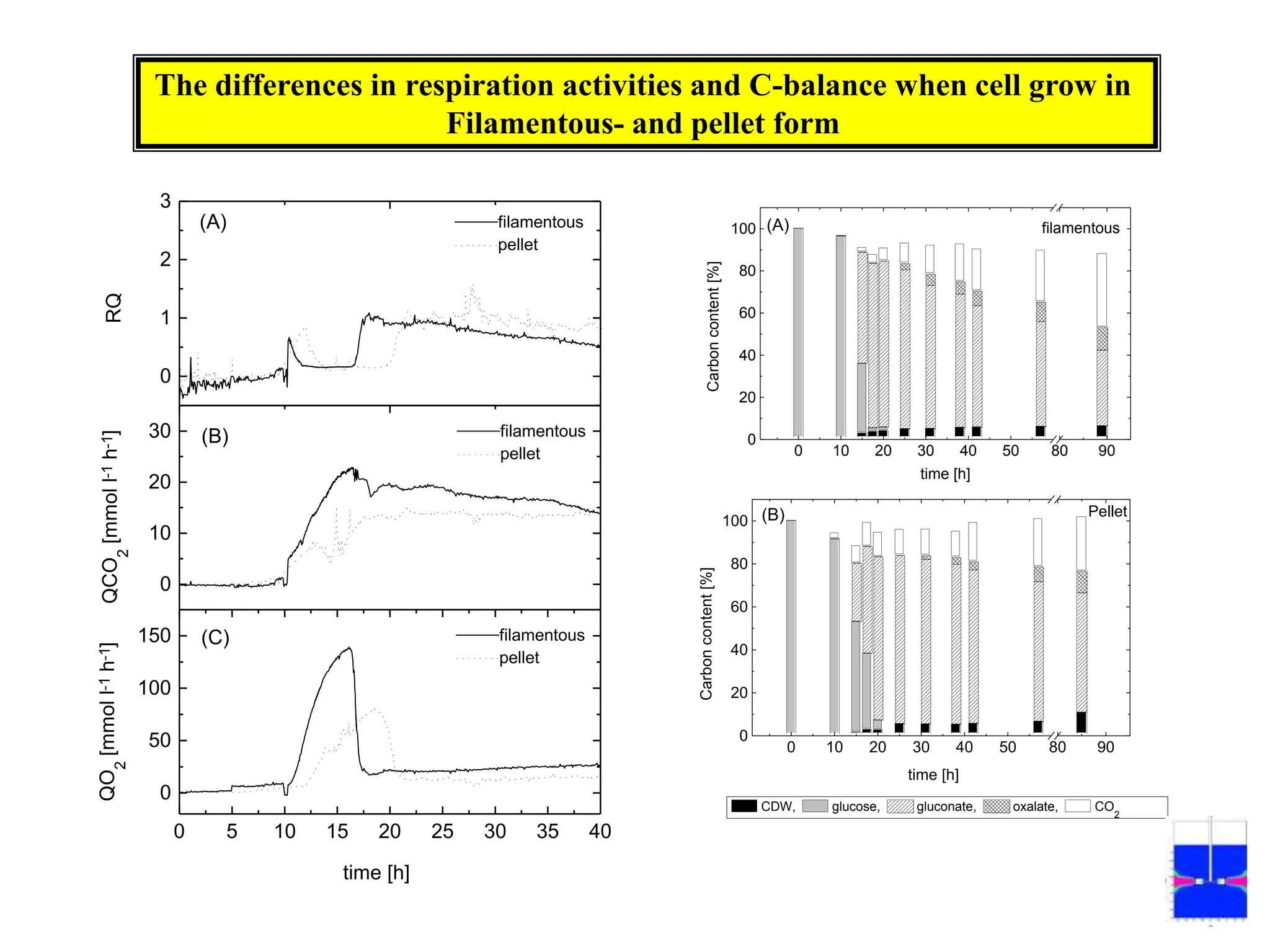
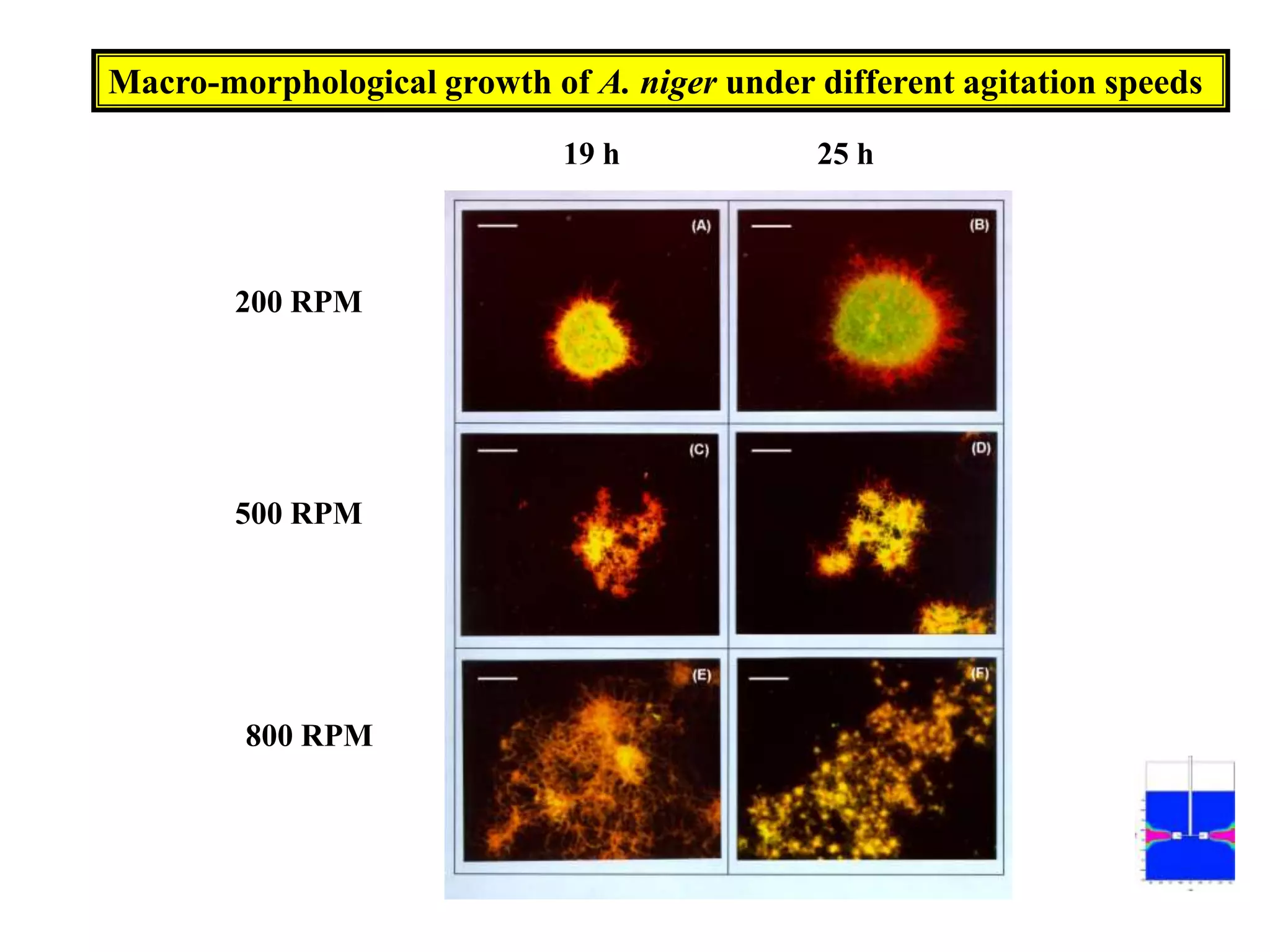
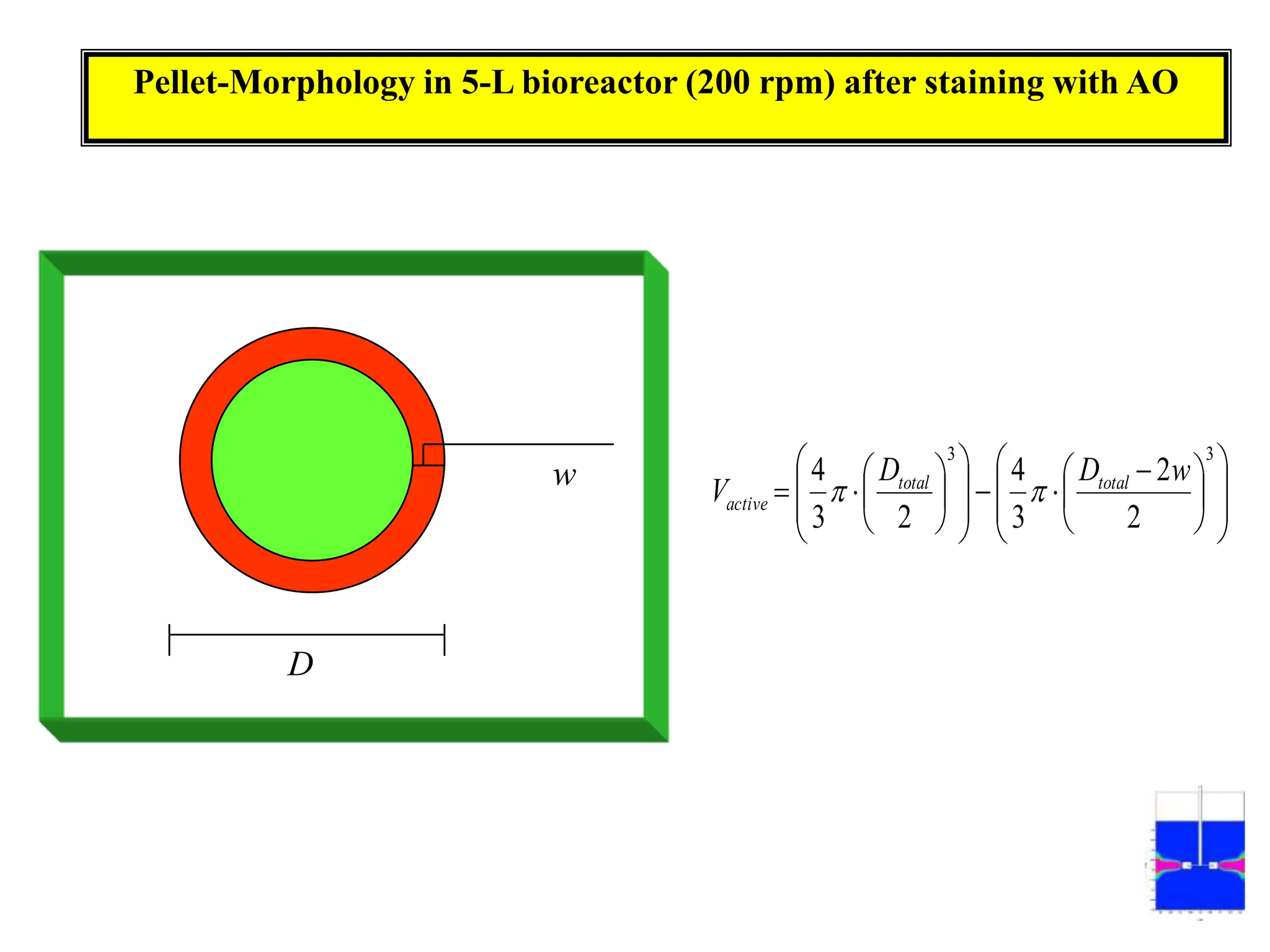



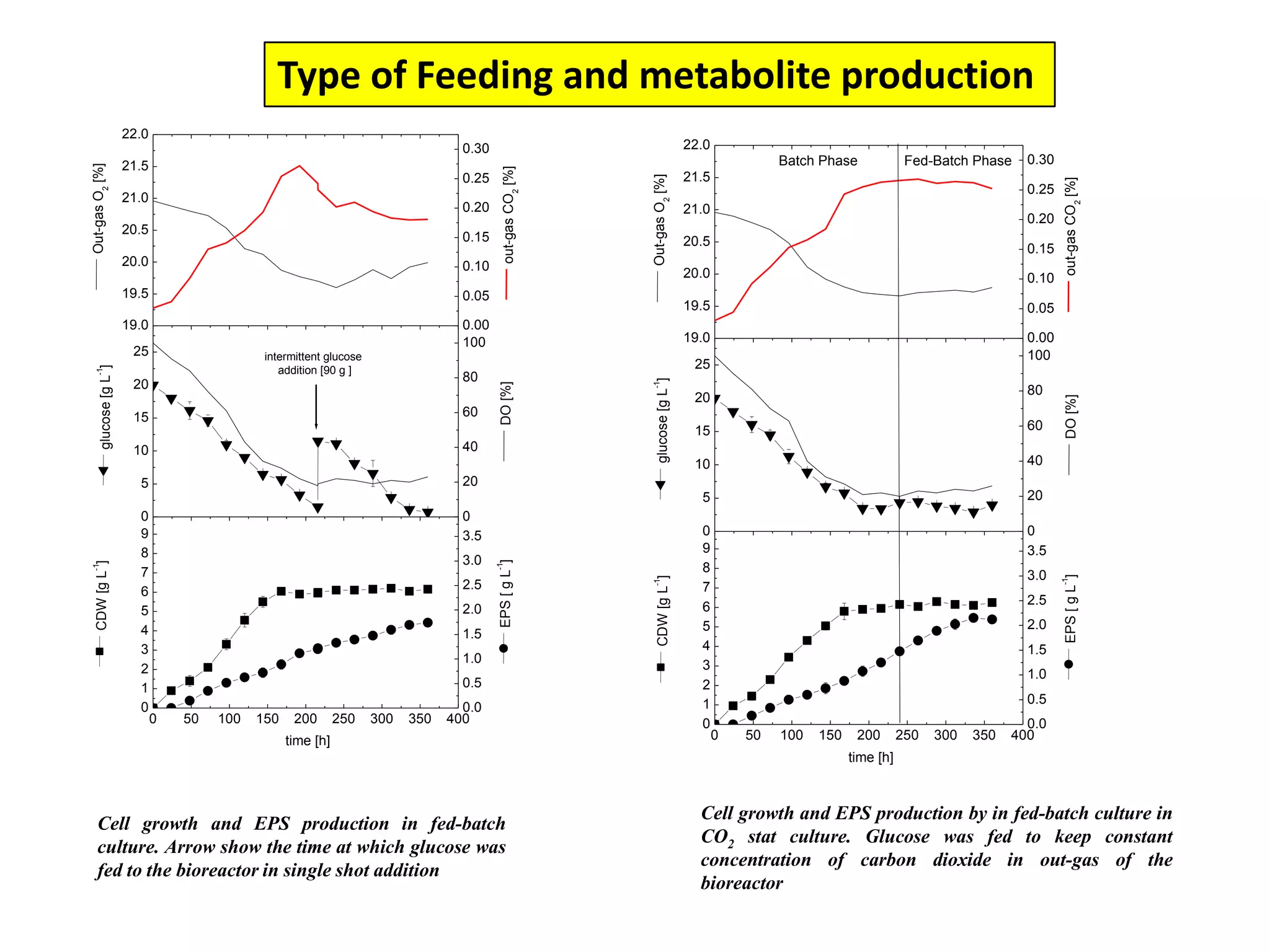
![Exponential feeding of substrate(s)Example:Fed-batch cultivation strategy (exponential feeding) for a recombinant strain of Asperigllusniger for glucose oxidase production. WhereMs Mass flow of substrate [g h-1]t Cultivation time [h]tF Start time of feeding phase [h]µset Adjusted specific growth rate [h-1]E Maintenance coefficient [g g-1 h-1]YX/S The biomass/substrate yield coefficient [g g-1]XF The biomass concentration at the start time of feeding phase [g]VL The culture volume [L]](https://image.slidesharecdn.com/lecture5-bioprocesstechnologyoperationmodeandscale-100716233917-phpapp02/75/Lecture-5-bioprocess-technology-operation-mode-and-scale-32-2048.jpg)



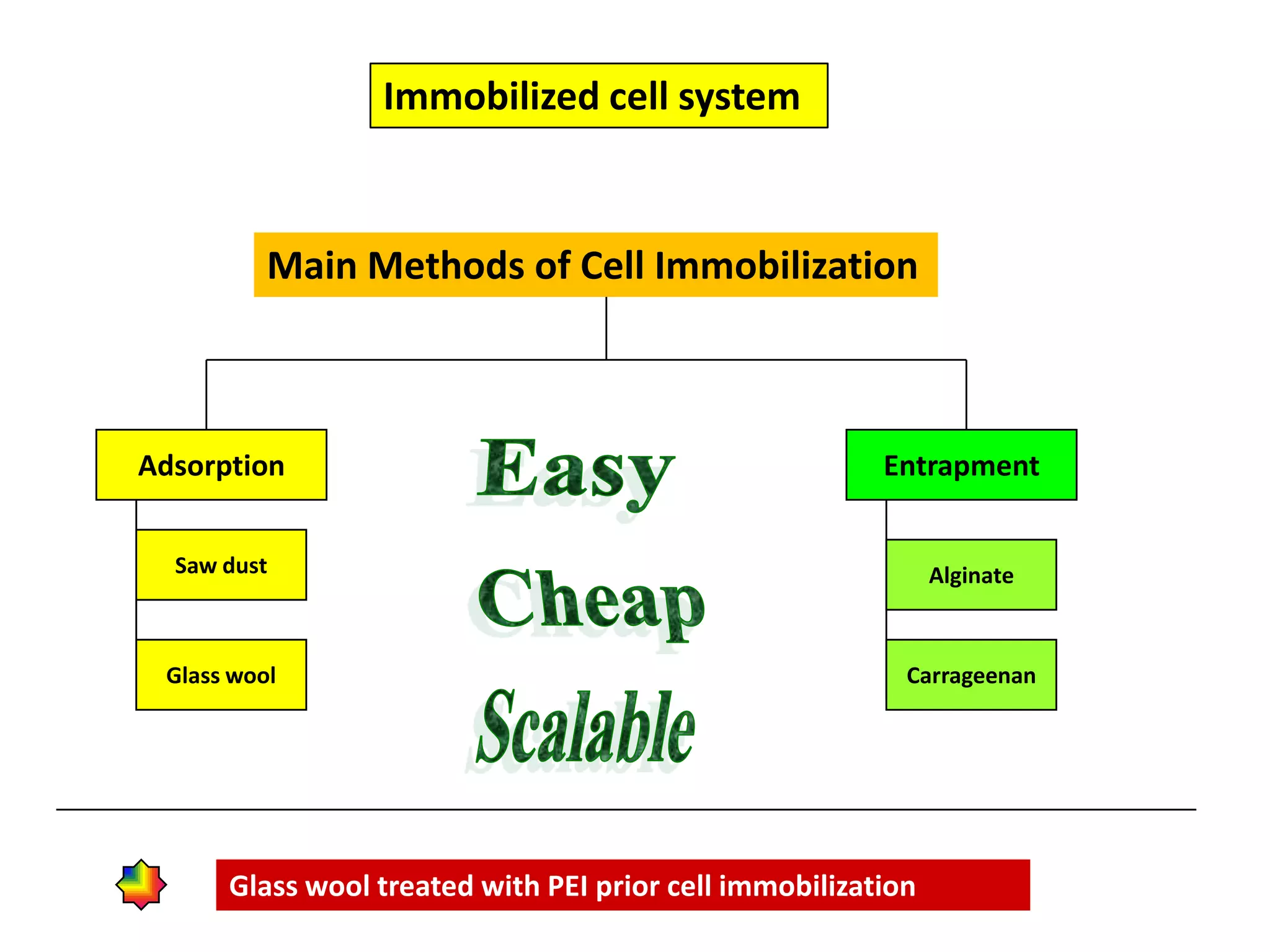
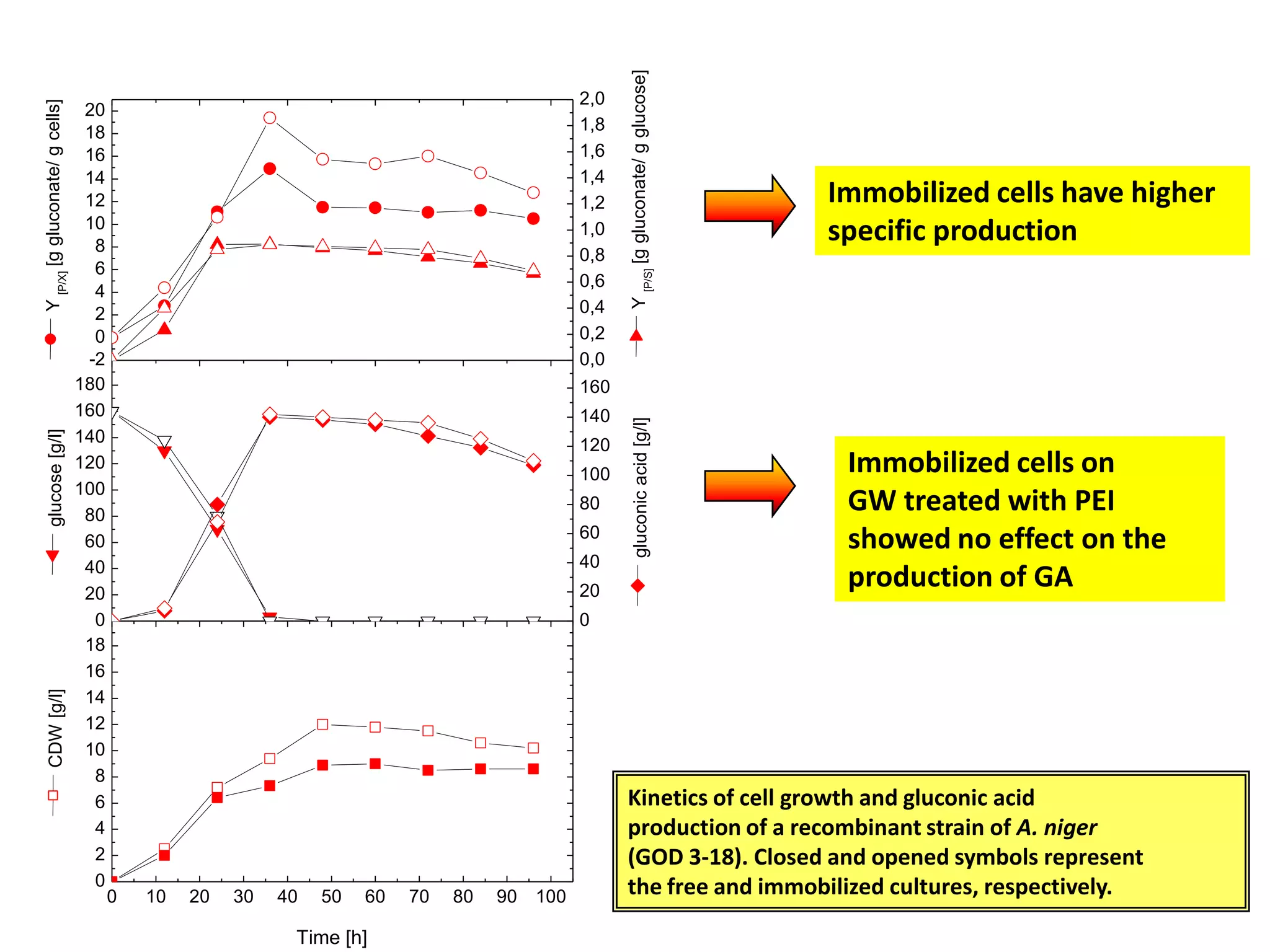
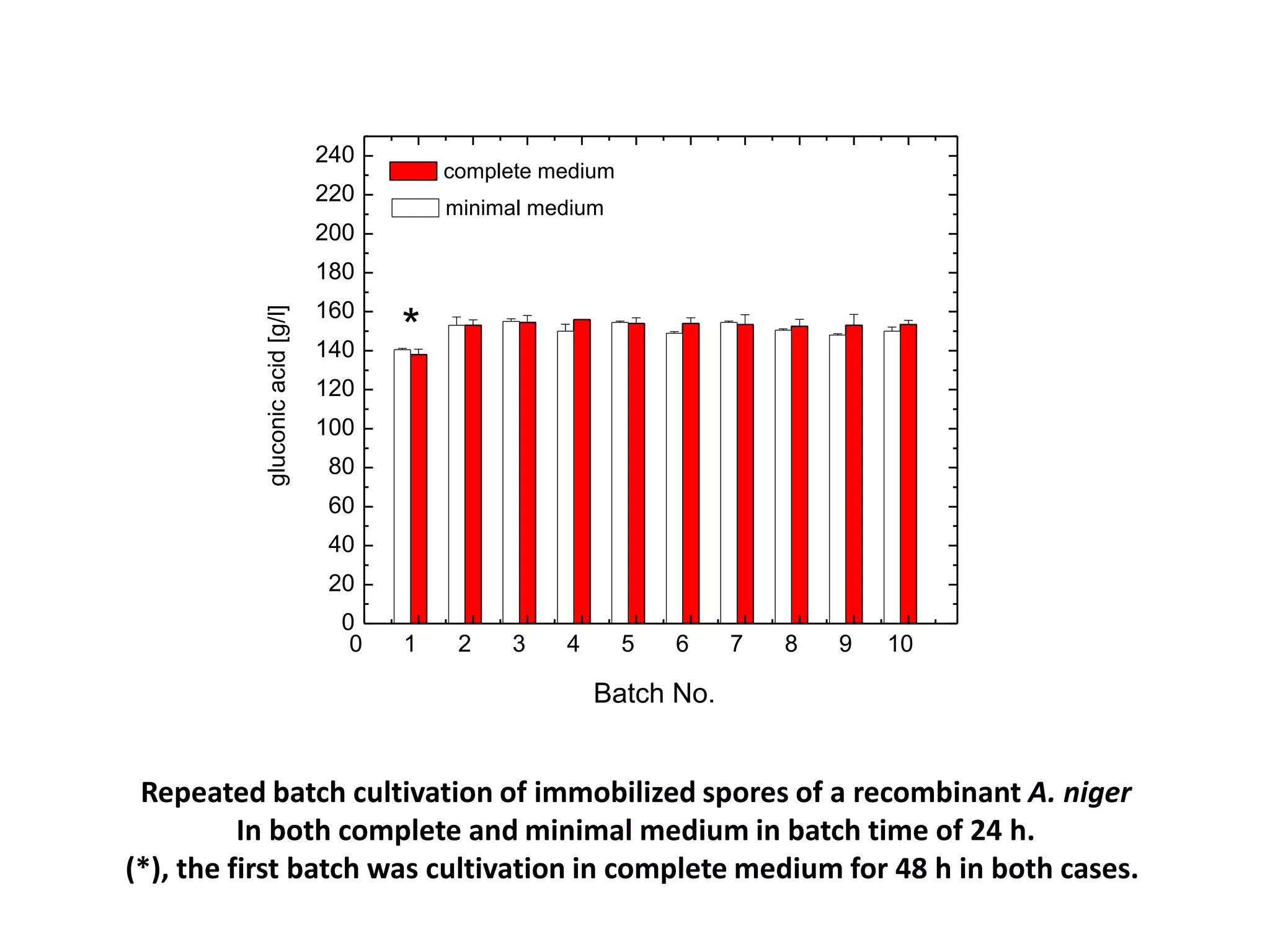
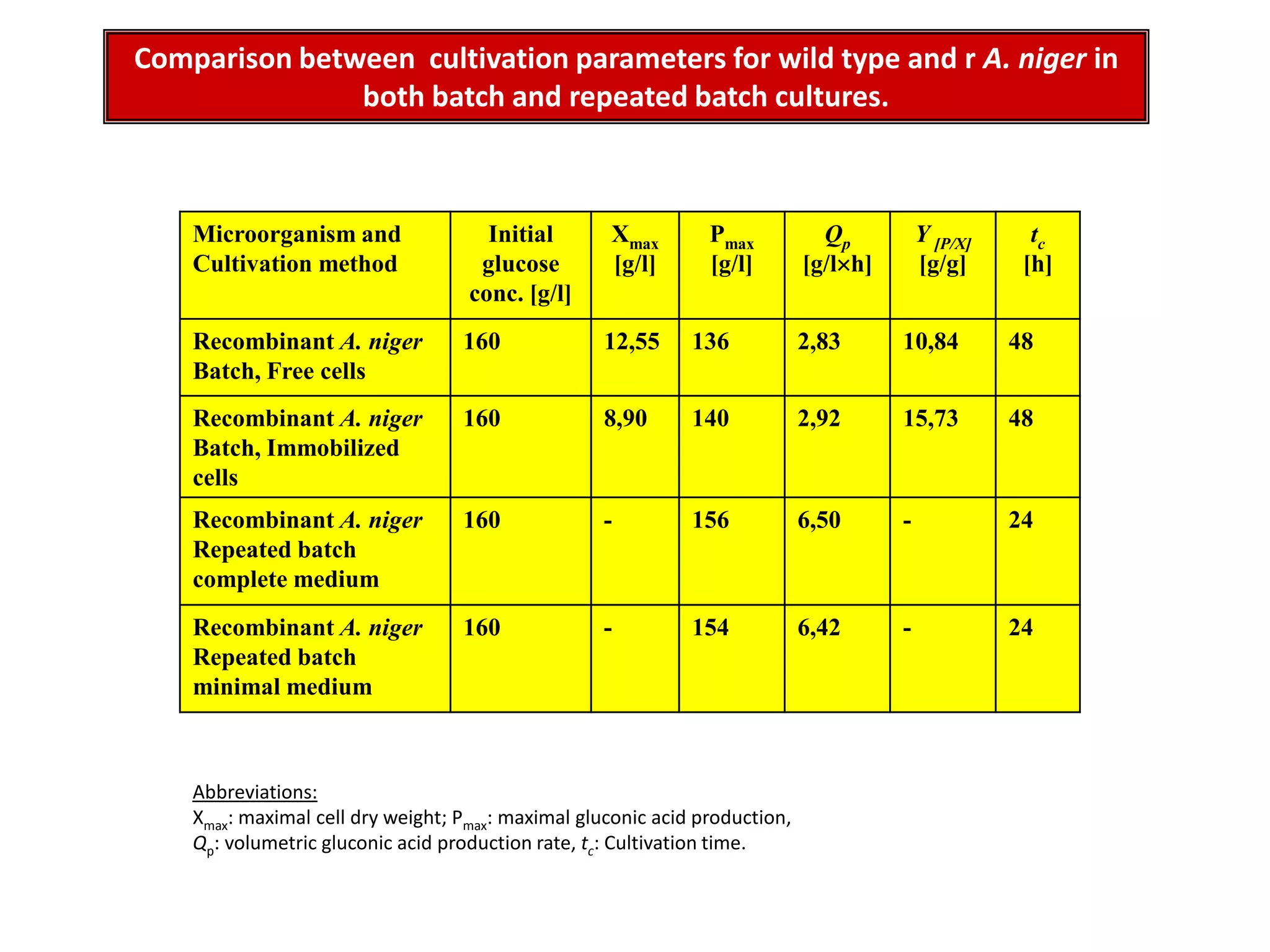
![Production medium for Immobilized cellsThe fermentation medium used for gluconic acid production By immobilized cells was of the following composition [g/l]: Complete medium Minimal mediumglucose, 160.0 160.0NaNO3, 3.0 1.0K2HPO4, 1.0 -MgSO4.7H2O, 0.5 0.2KCl, 0.5 -FeSO4.7H2O, 0.01 -Yeast extract, 2.0 -The pH of medium was adjusted to 5.5](https://image.slidesharecdn.com/lecture5-bioprocesstechnologyoperationmodeandscale-100716233917-phpapp02/75/Lecture-5-bioprocess-technology-operation-mode-and-scale-44-2048.jpg)