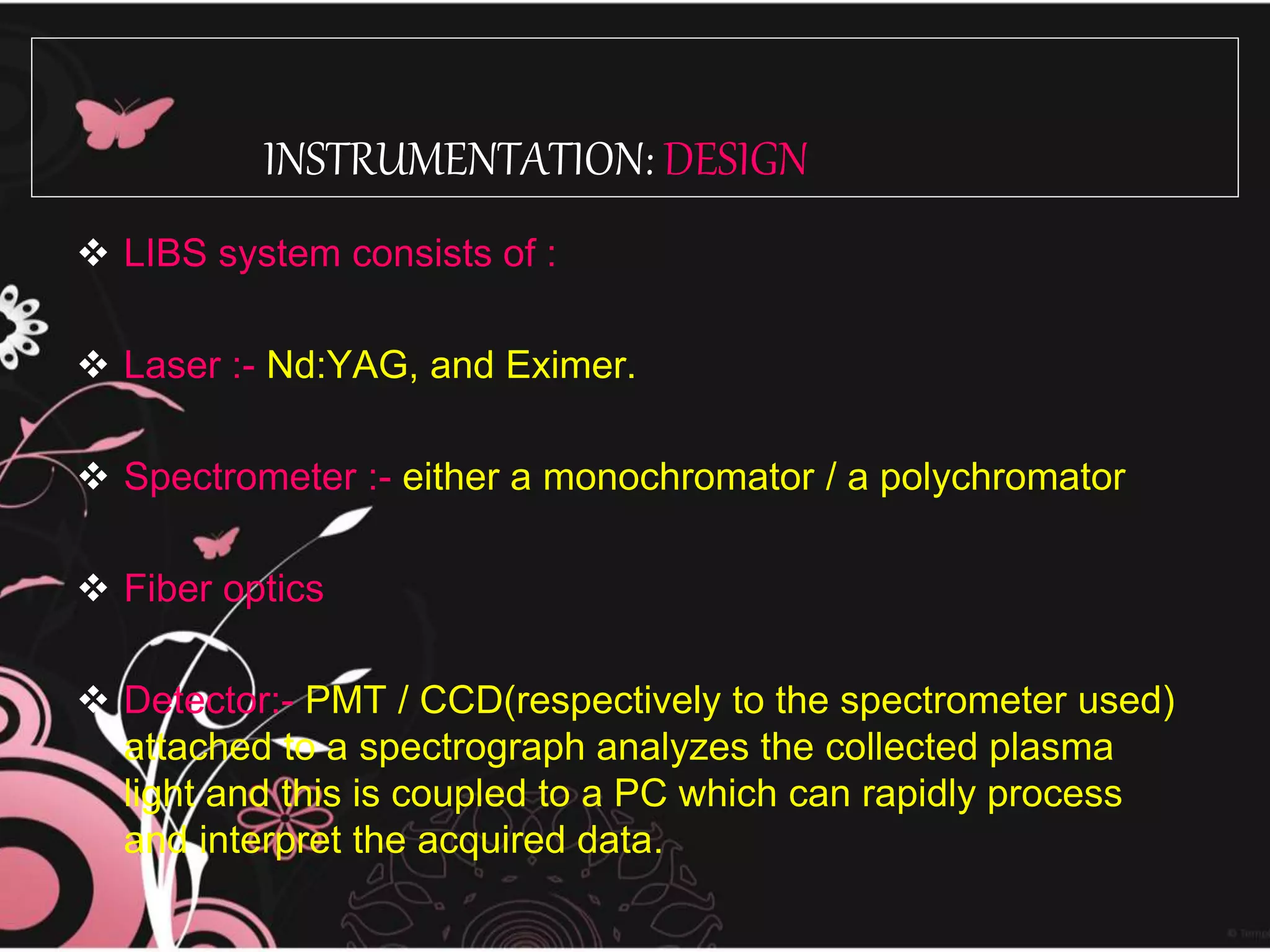
LIBS uses a highly energetic laser pulse to induce breakdown of a sample's surface, atomizing and exciting it. The spectrometer then analyzes the light emitted from the laser-induced plasma. LIBS allows for rapid, multi-elemental analysis of solids, liquids, and gases with little to no sample preparation. It has applications in pharmaceutical analysis, environmental monitoring, metallurgy, and more. Recent developments have improved LIBS sensitivity and enabled techniques like depth profiling of multilayer samples.