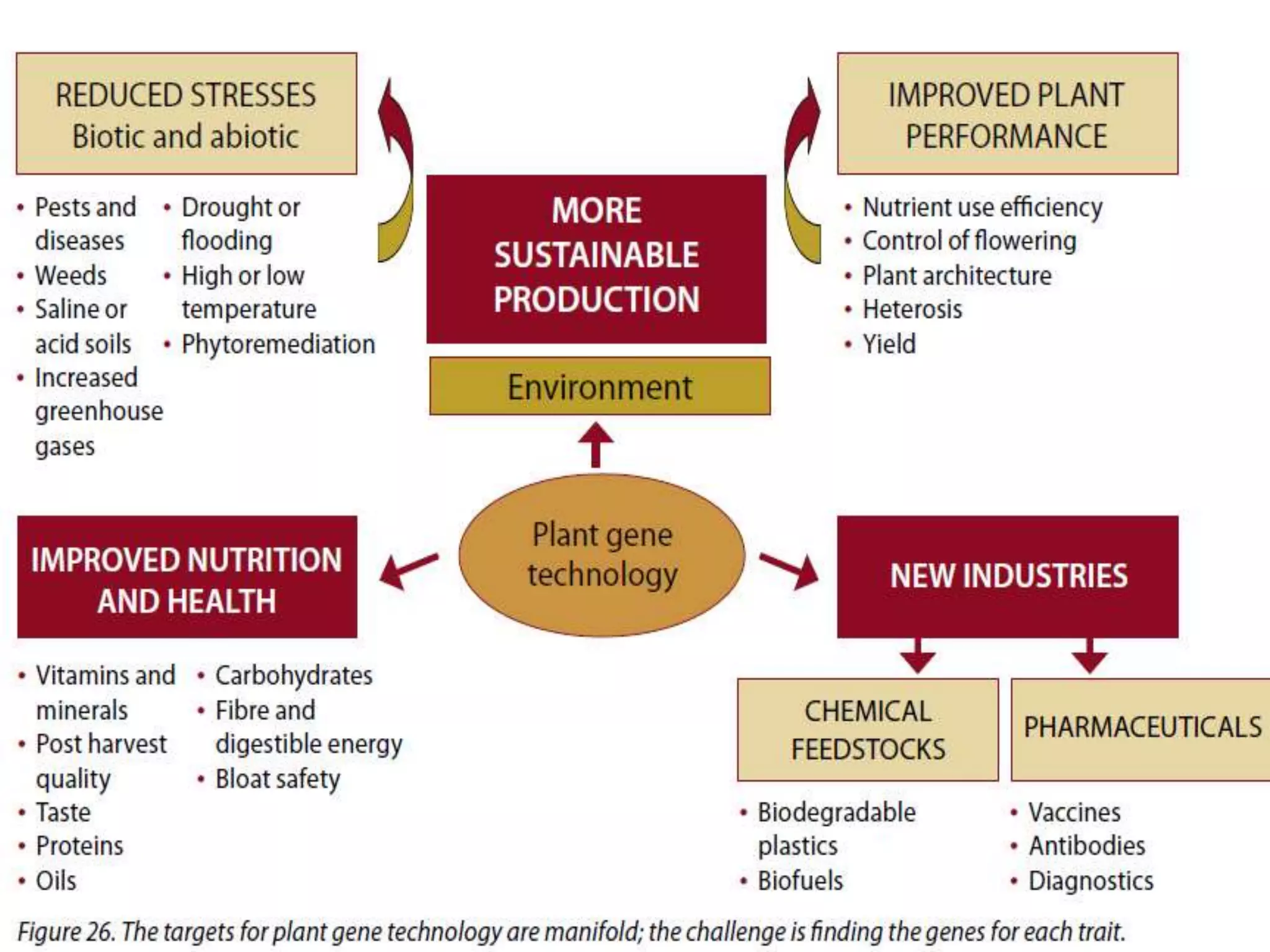
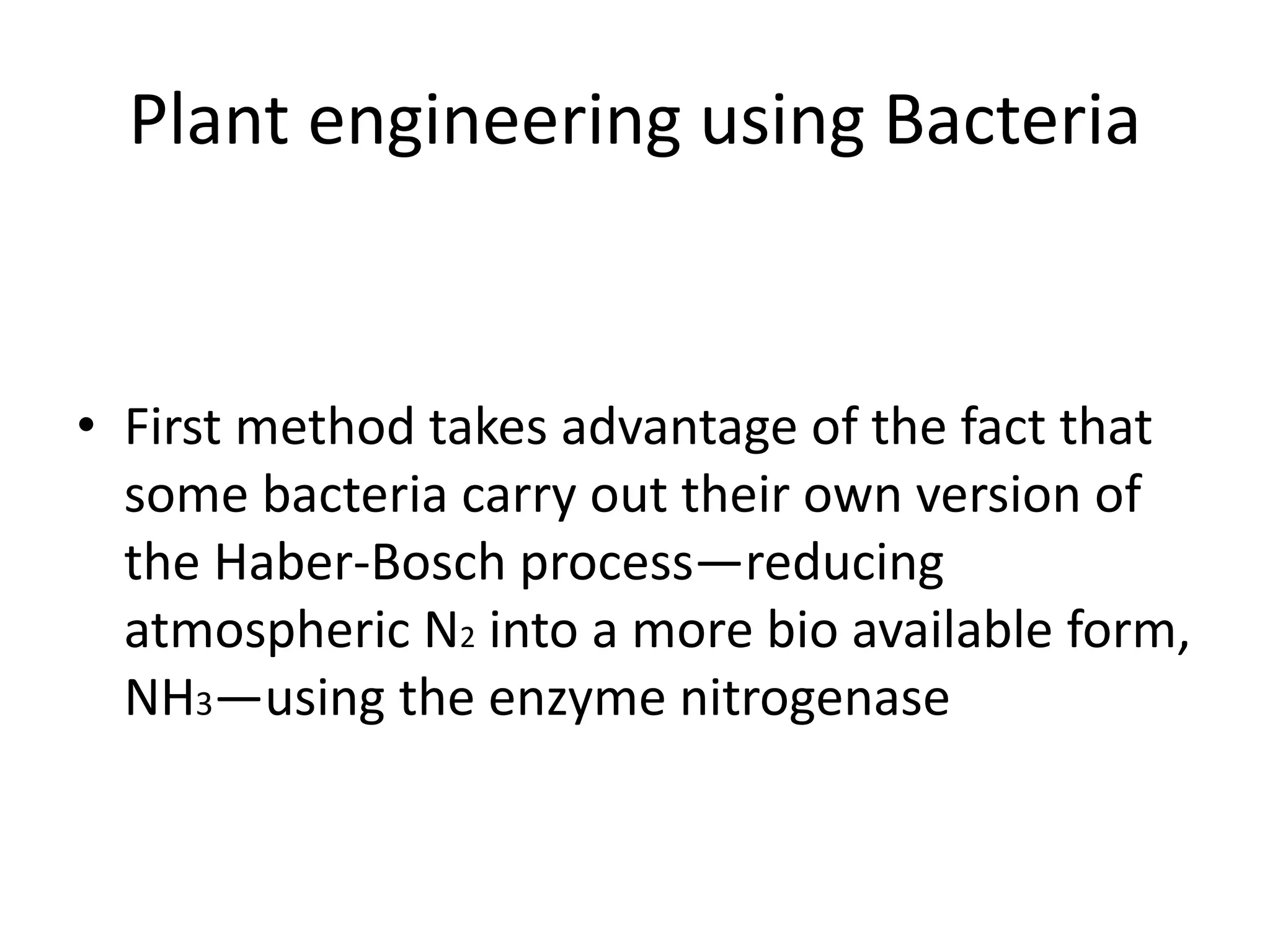
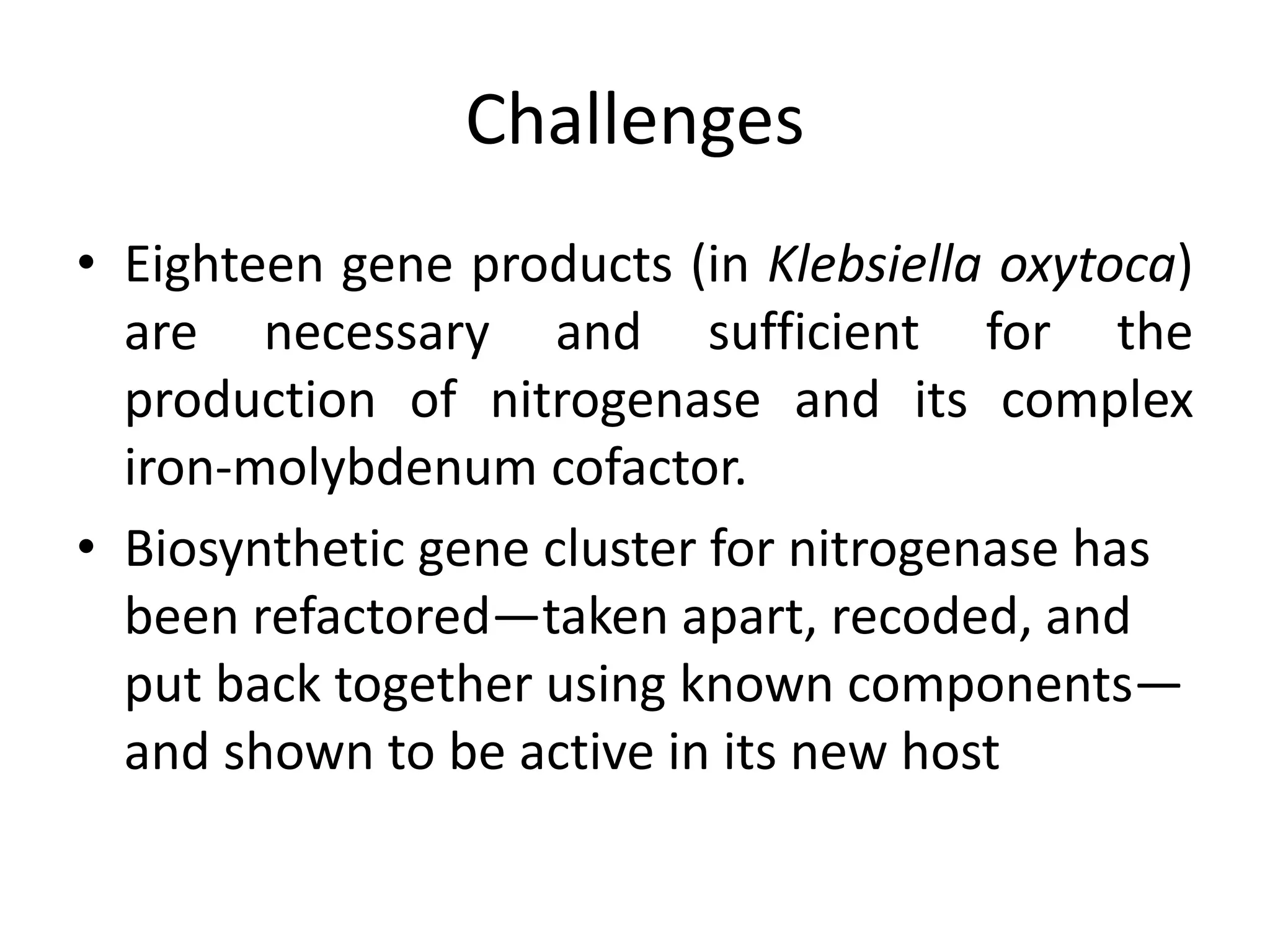
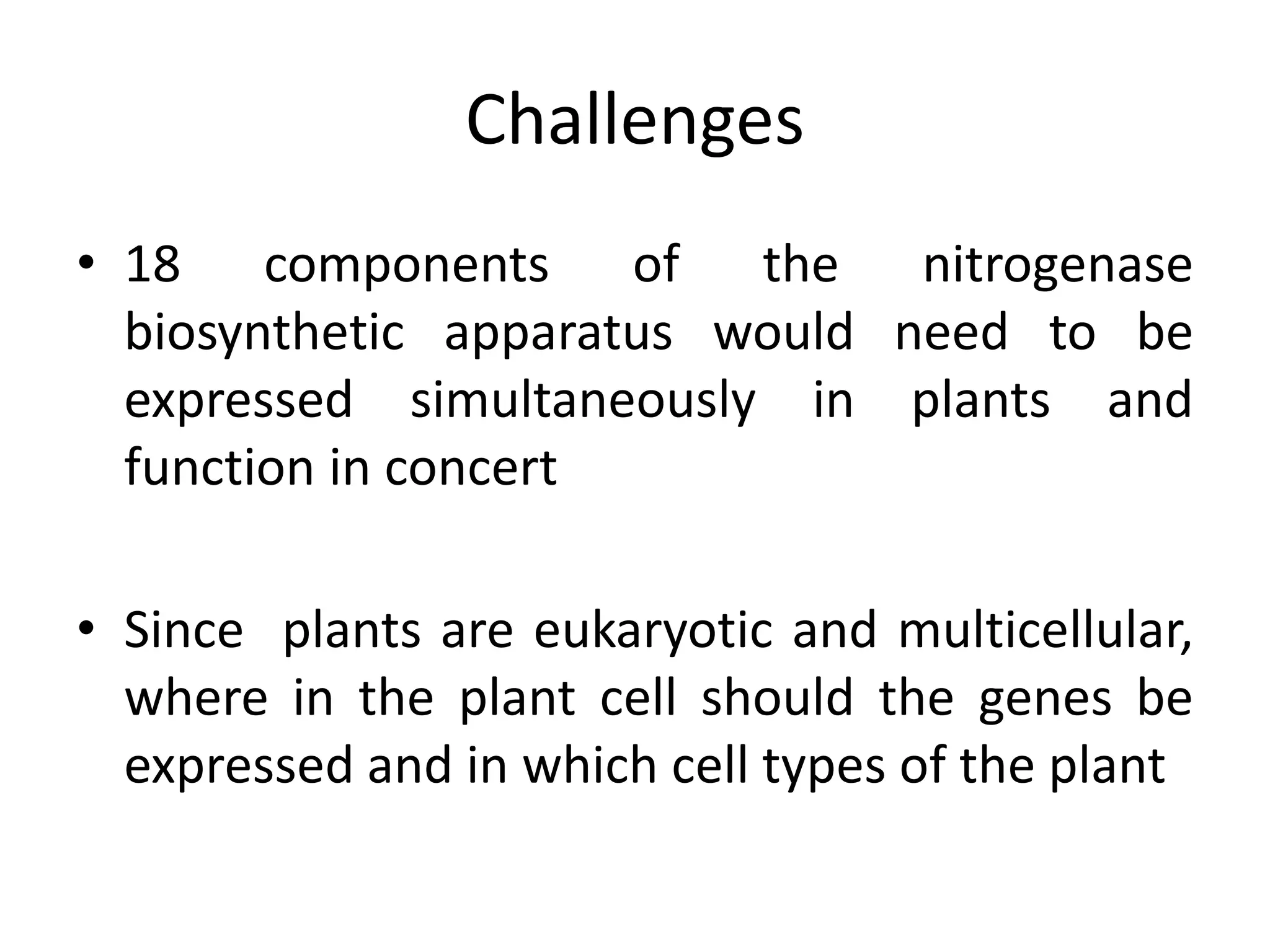
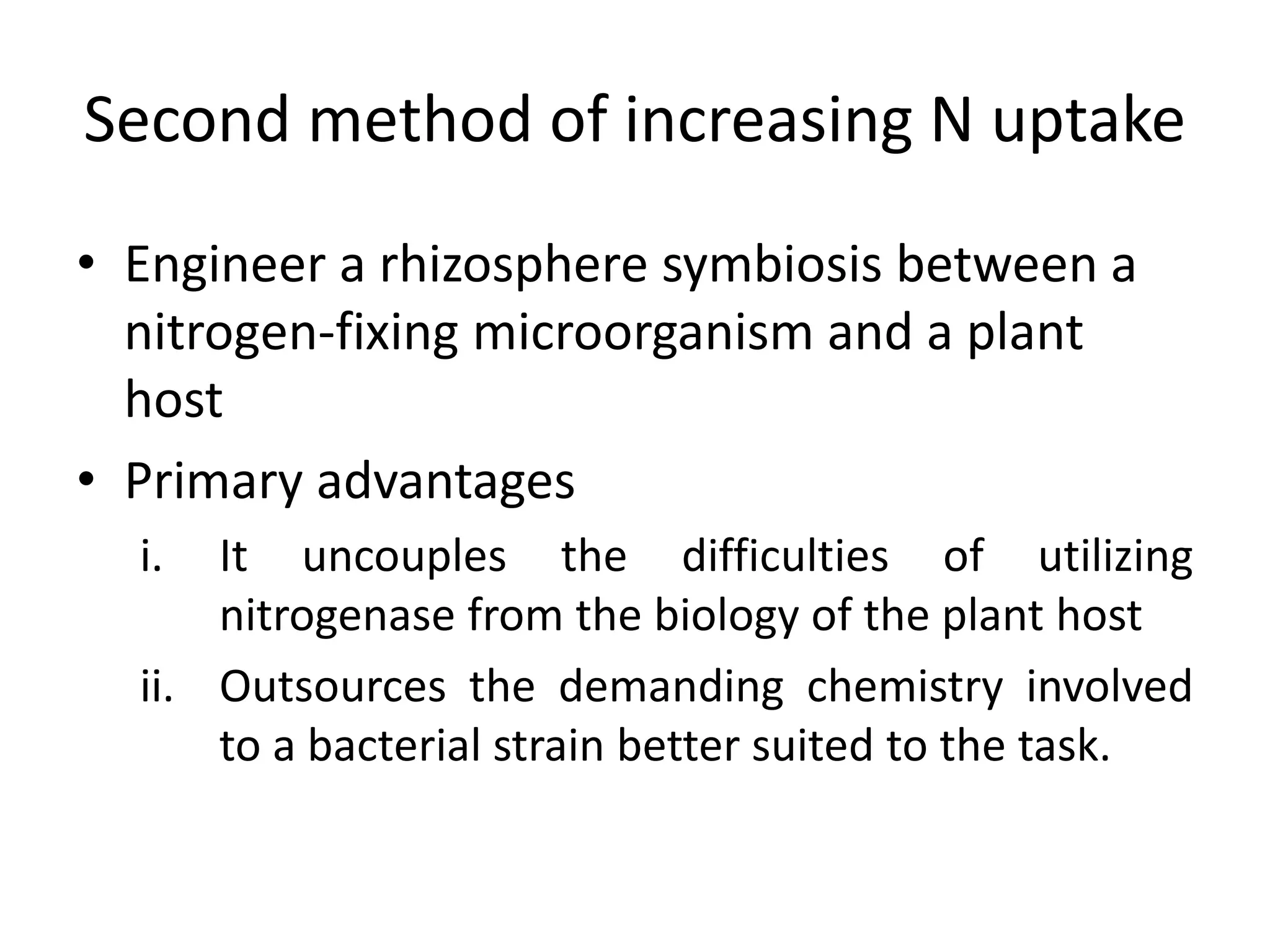
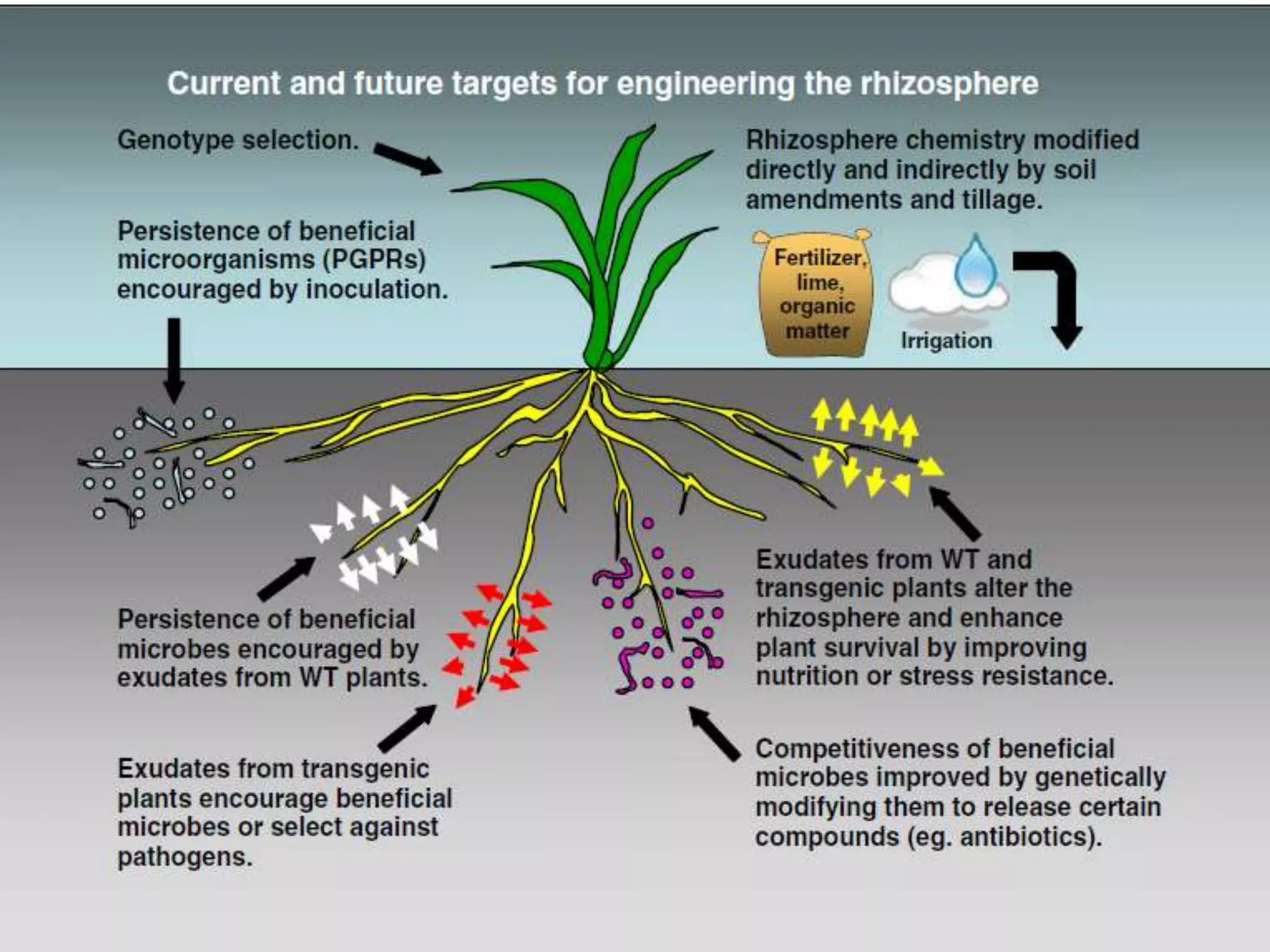
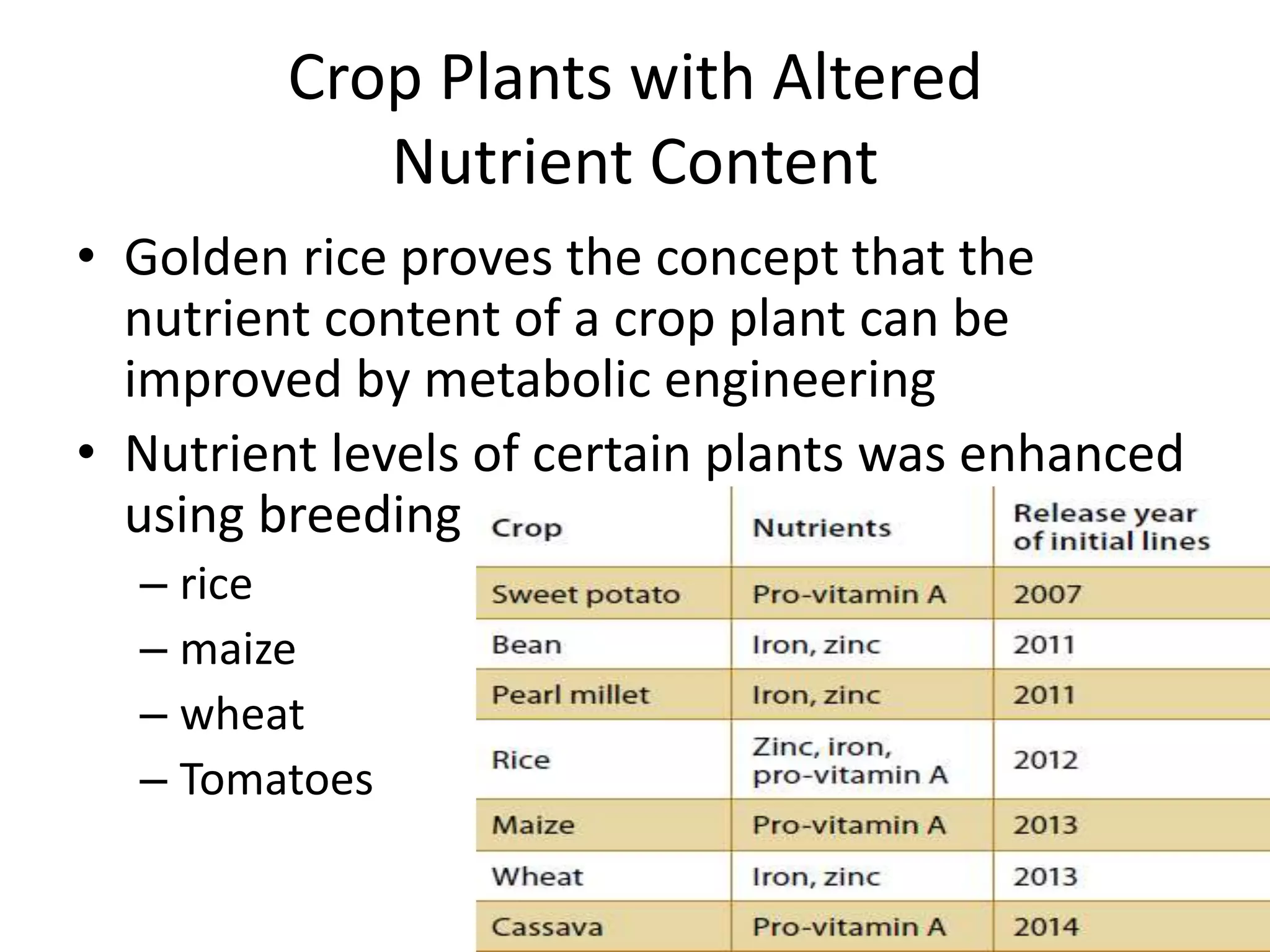
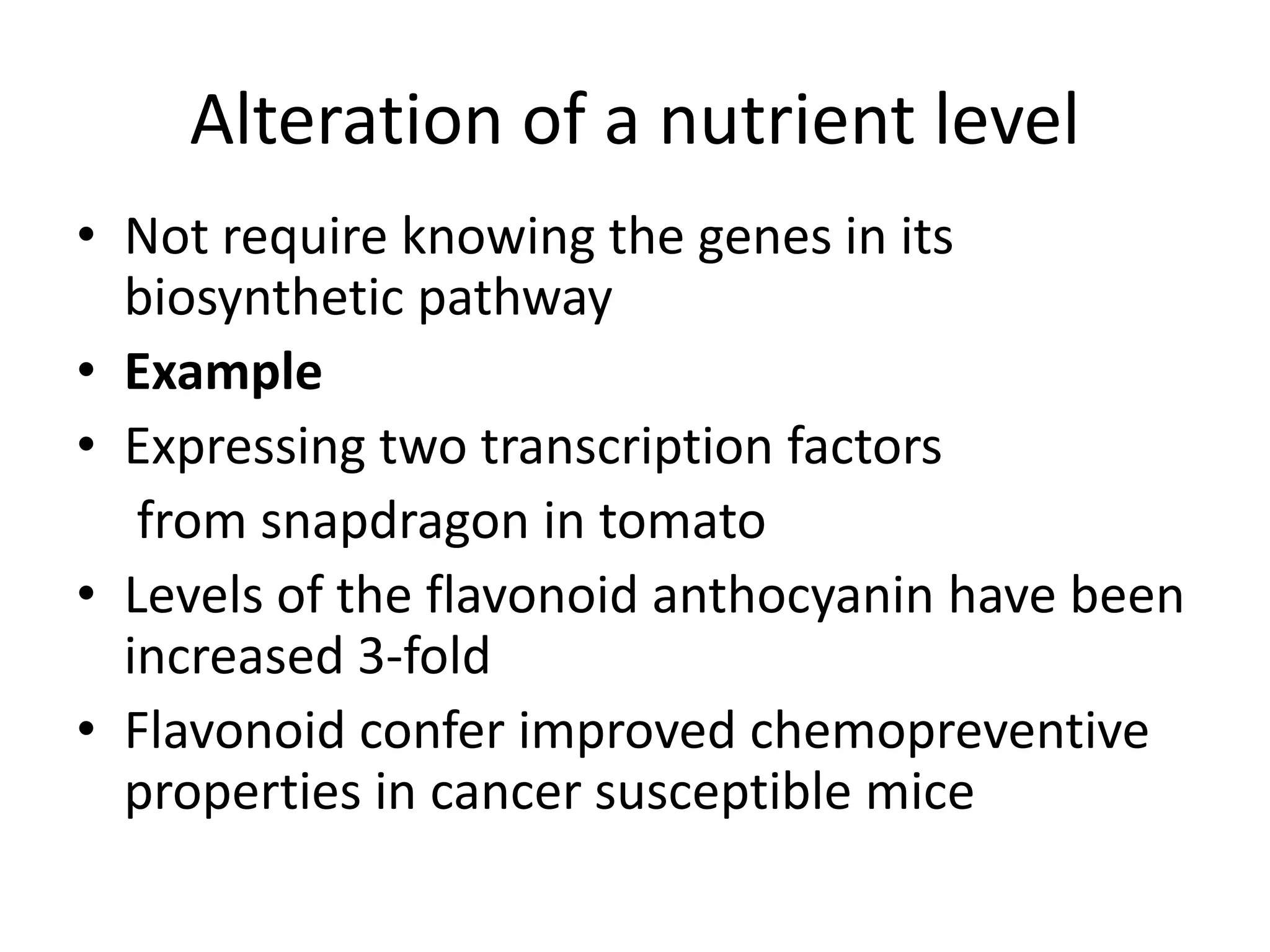
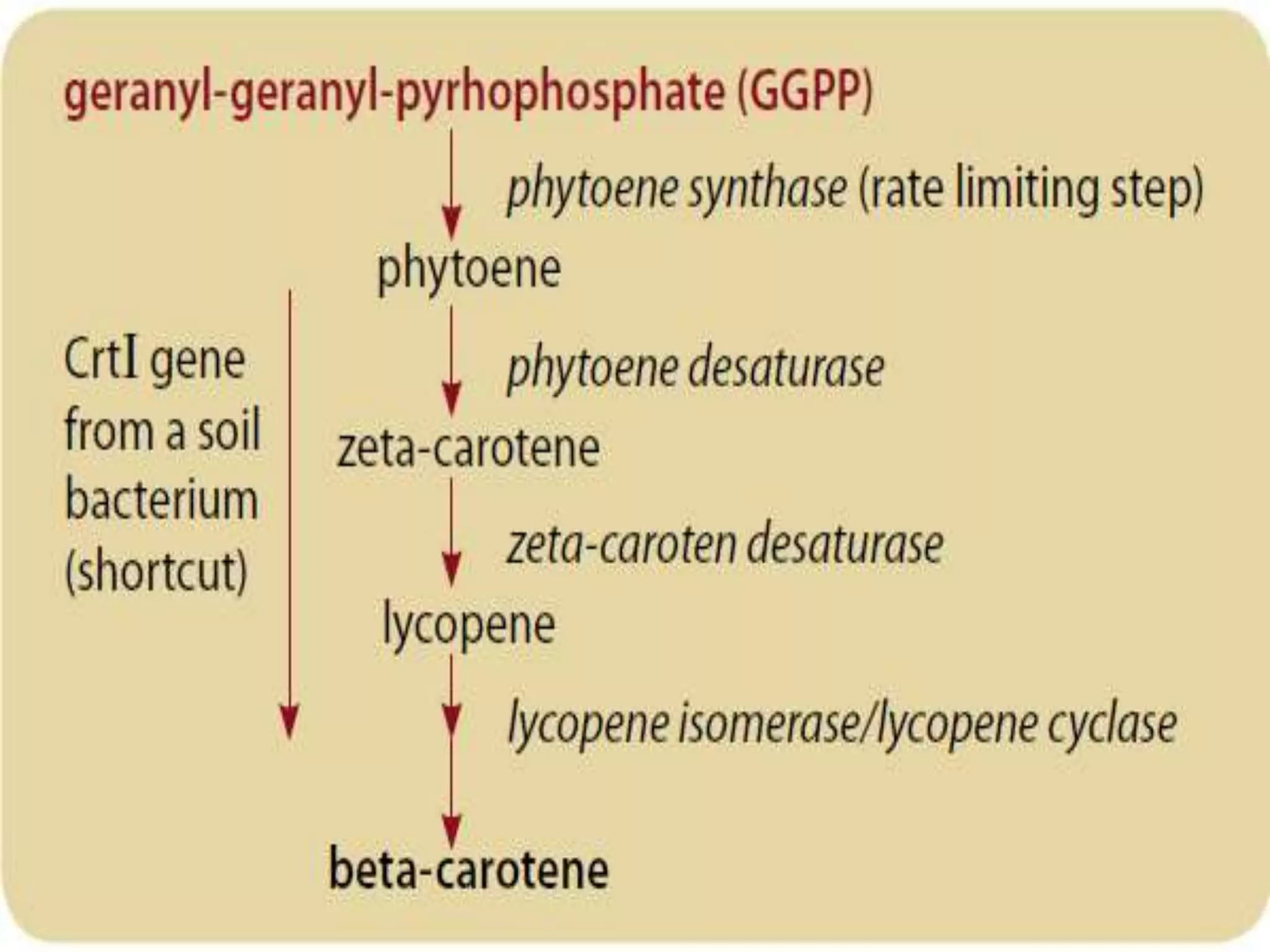
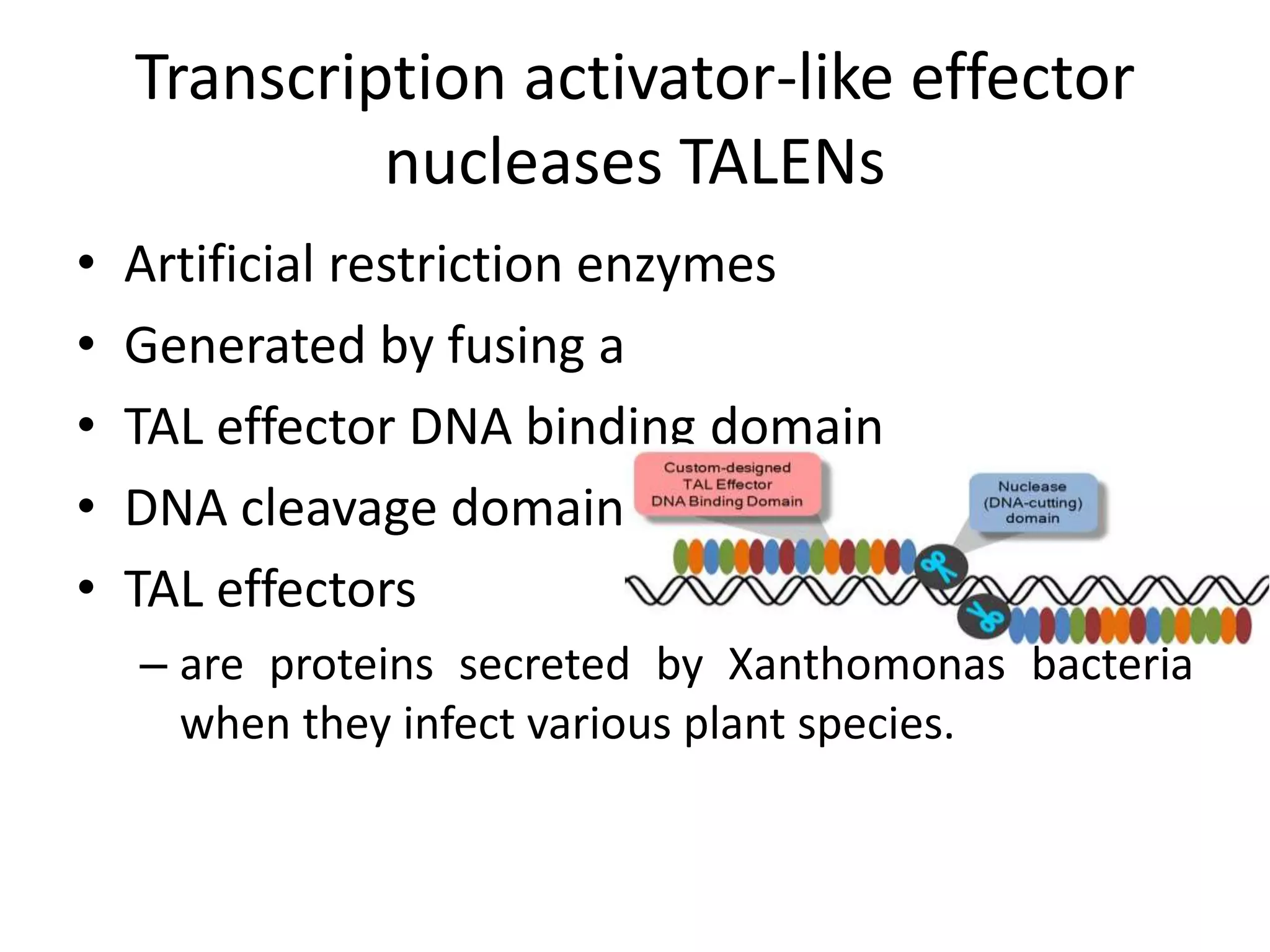
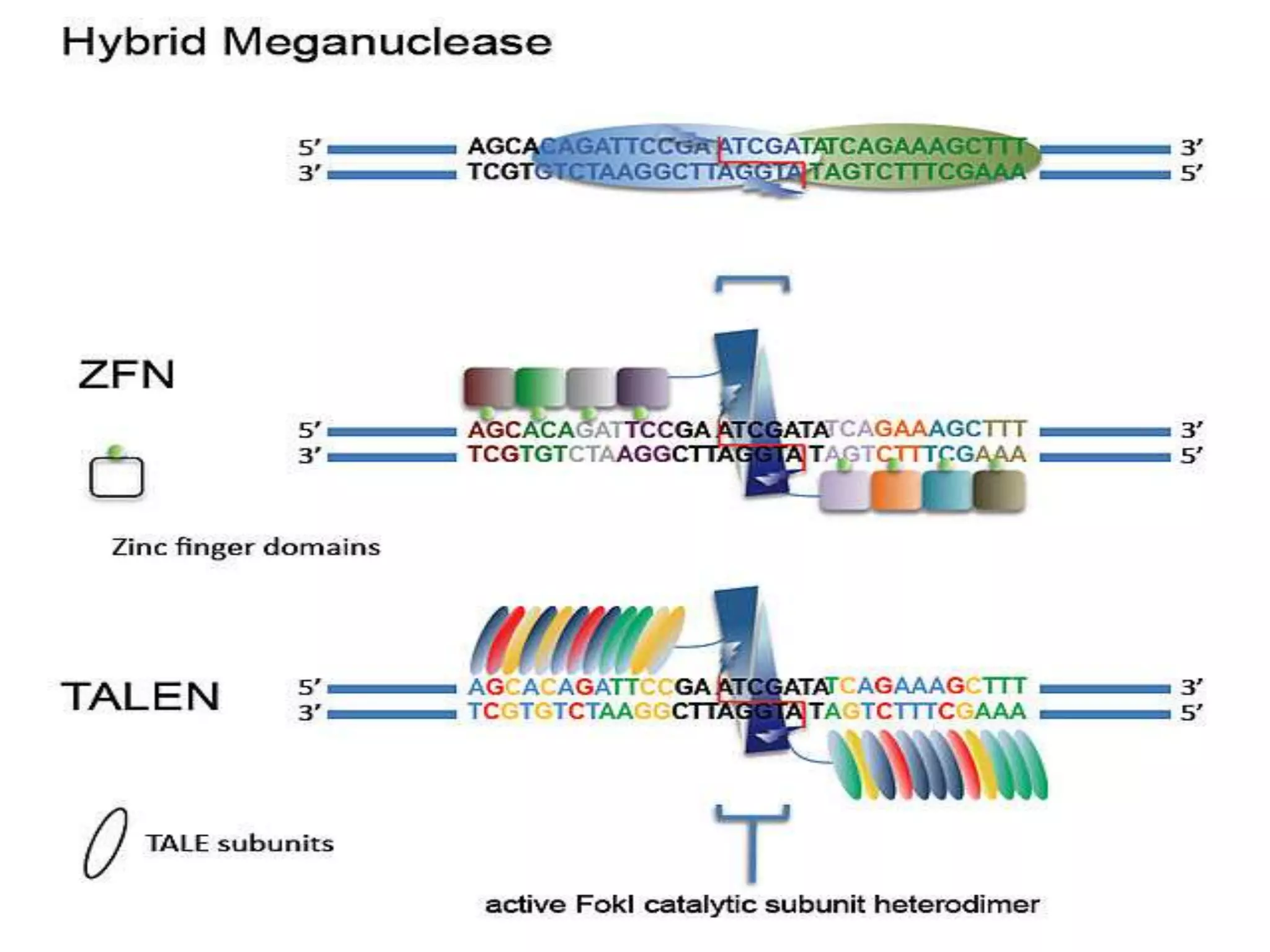
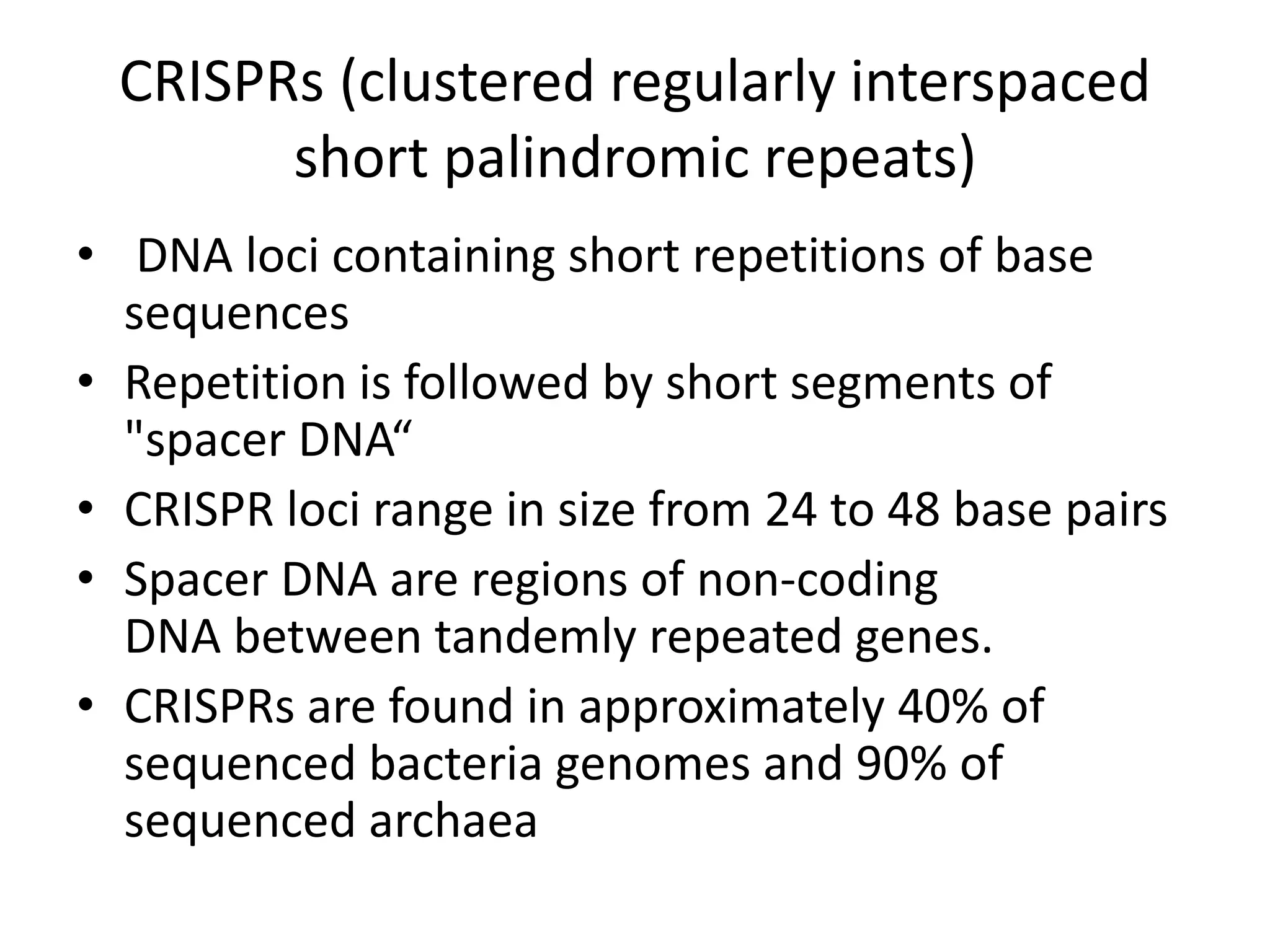
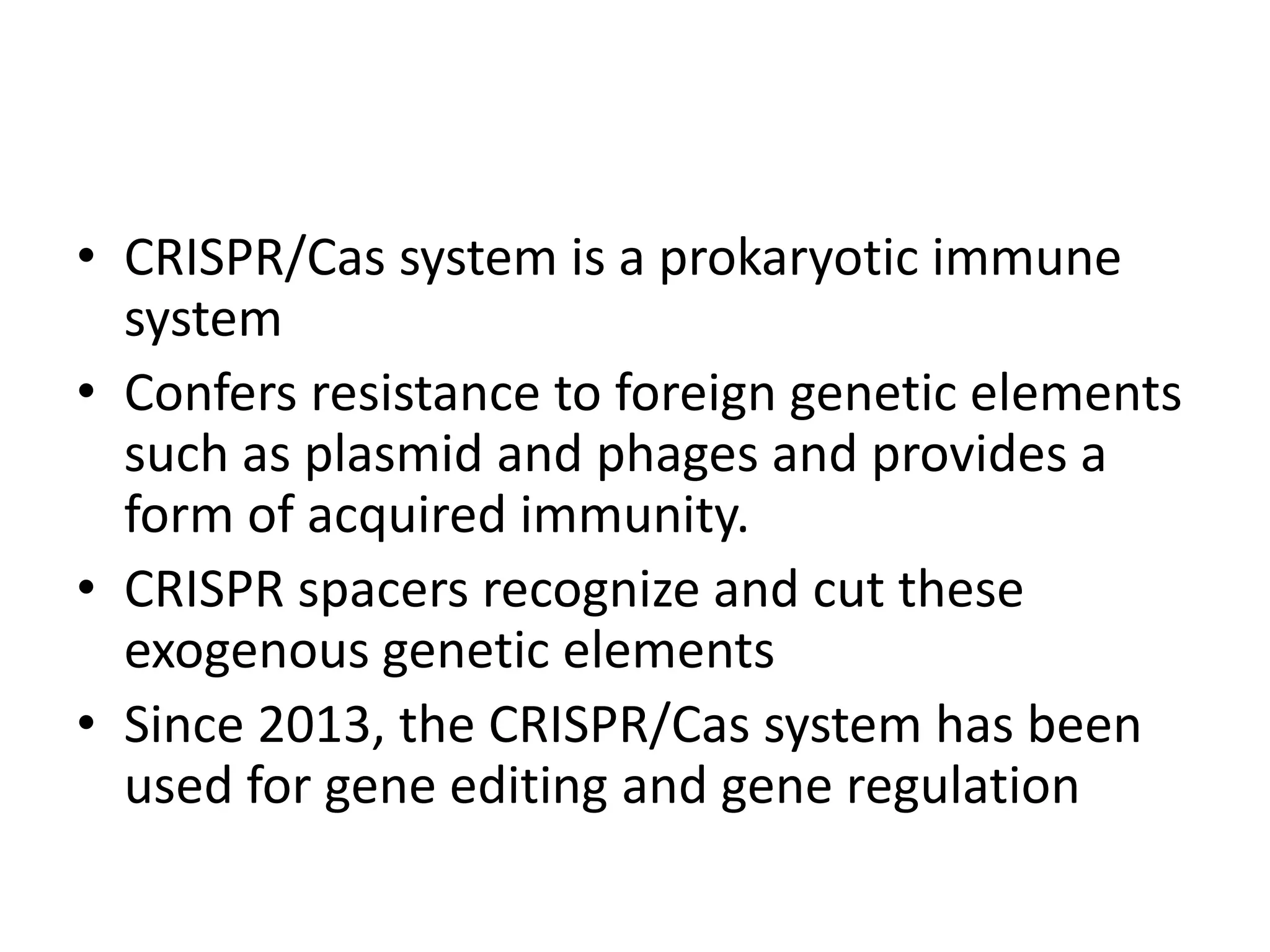
This document discusses key applications of plant metabolic engineering, including enabling plants to fix their own nitrogen, altering nutrient content of crop plants, enhancing photosynthetic efficiency, and using plants for biofuel production. Some of the challenges discussed are expressing the large number of genes required for nitrogen fixation in plants, developing efficient nutrient exchange in plant-microbe symbiosis, and modifying lignin in plants to more easily access cellulose for biofuels while avoiding negative impacts on growth.