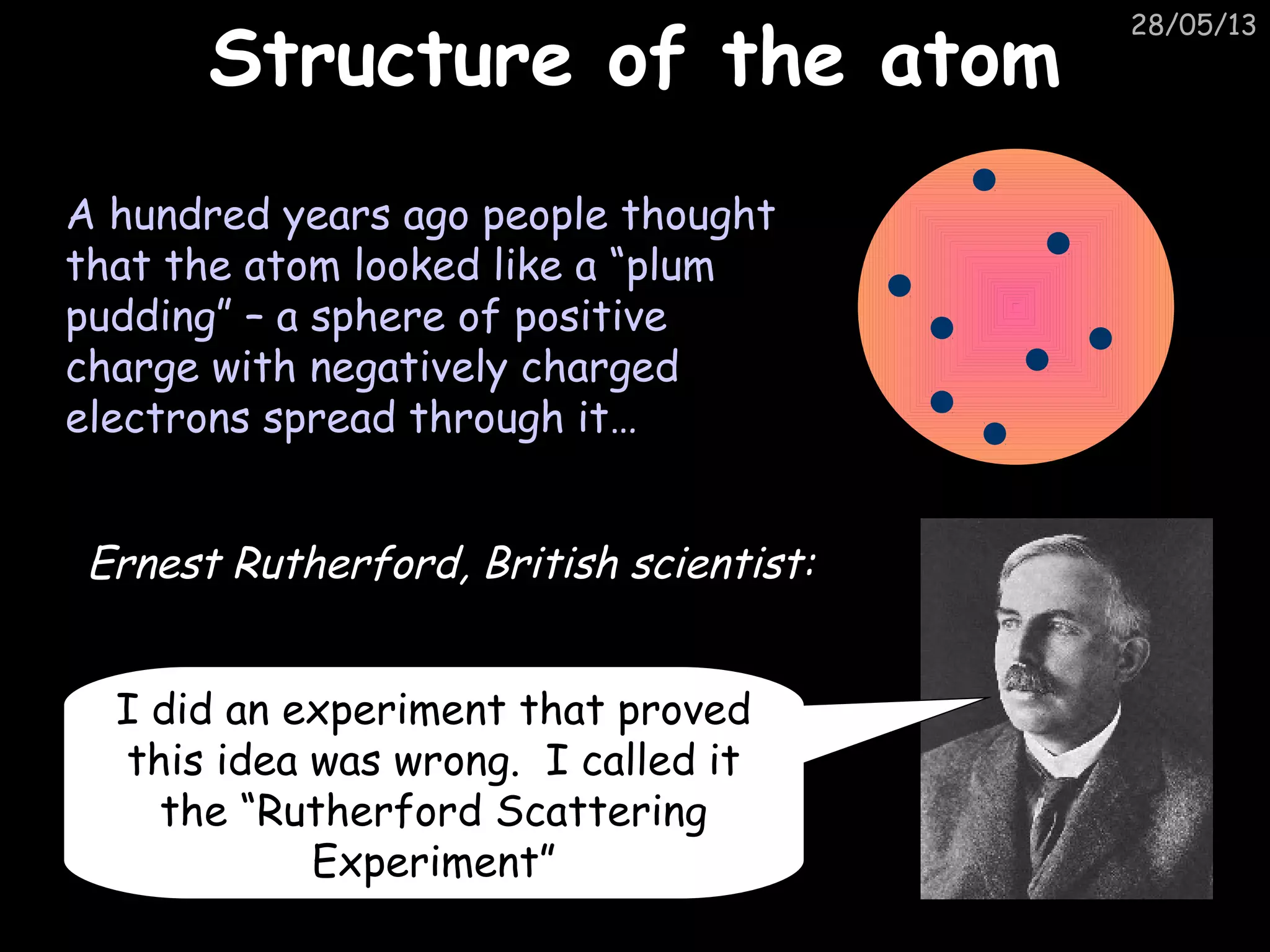
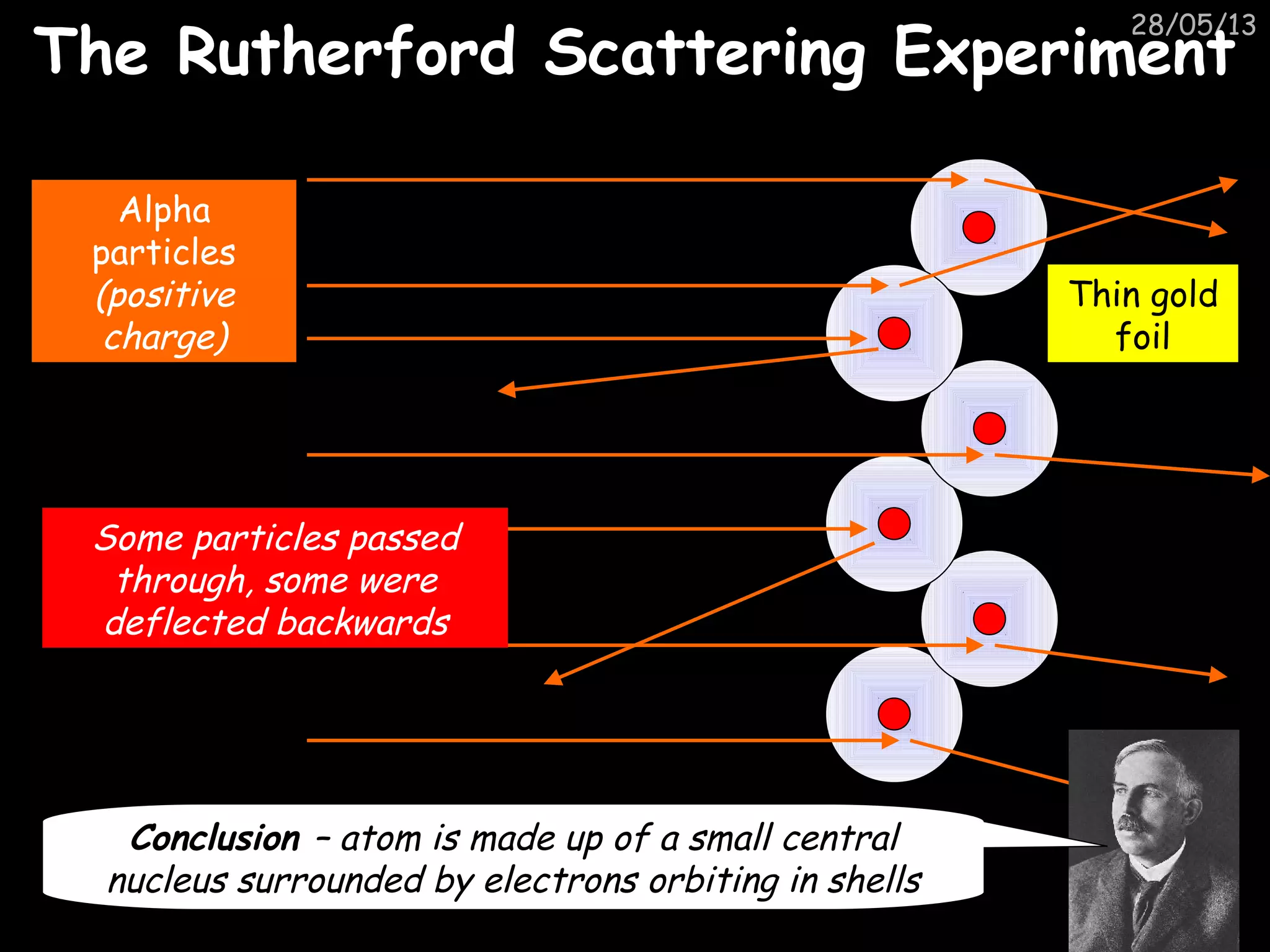
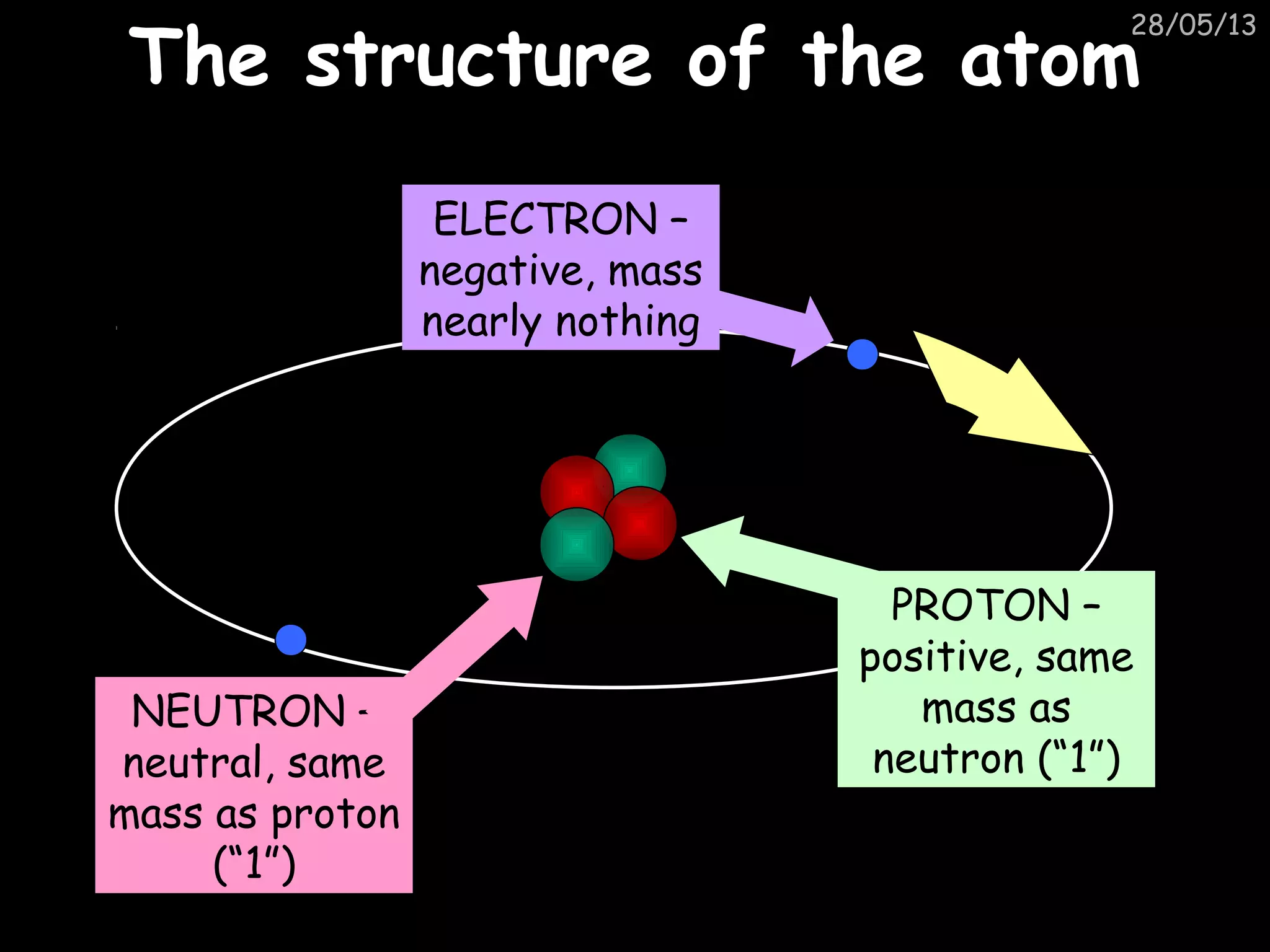
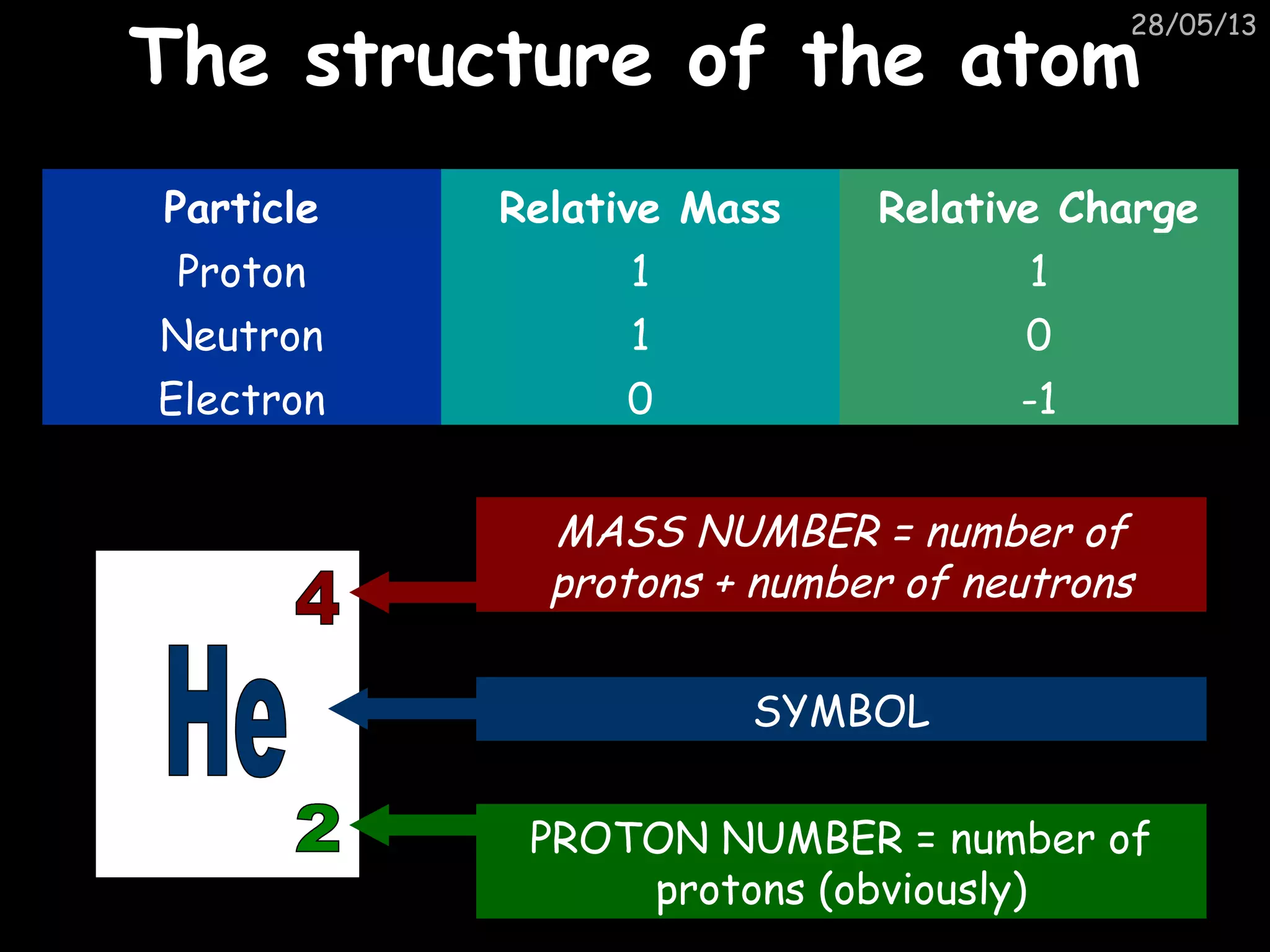
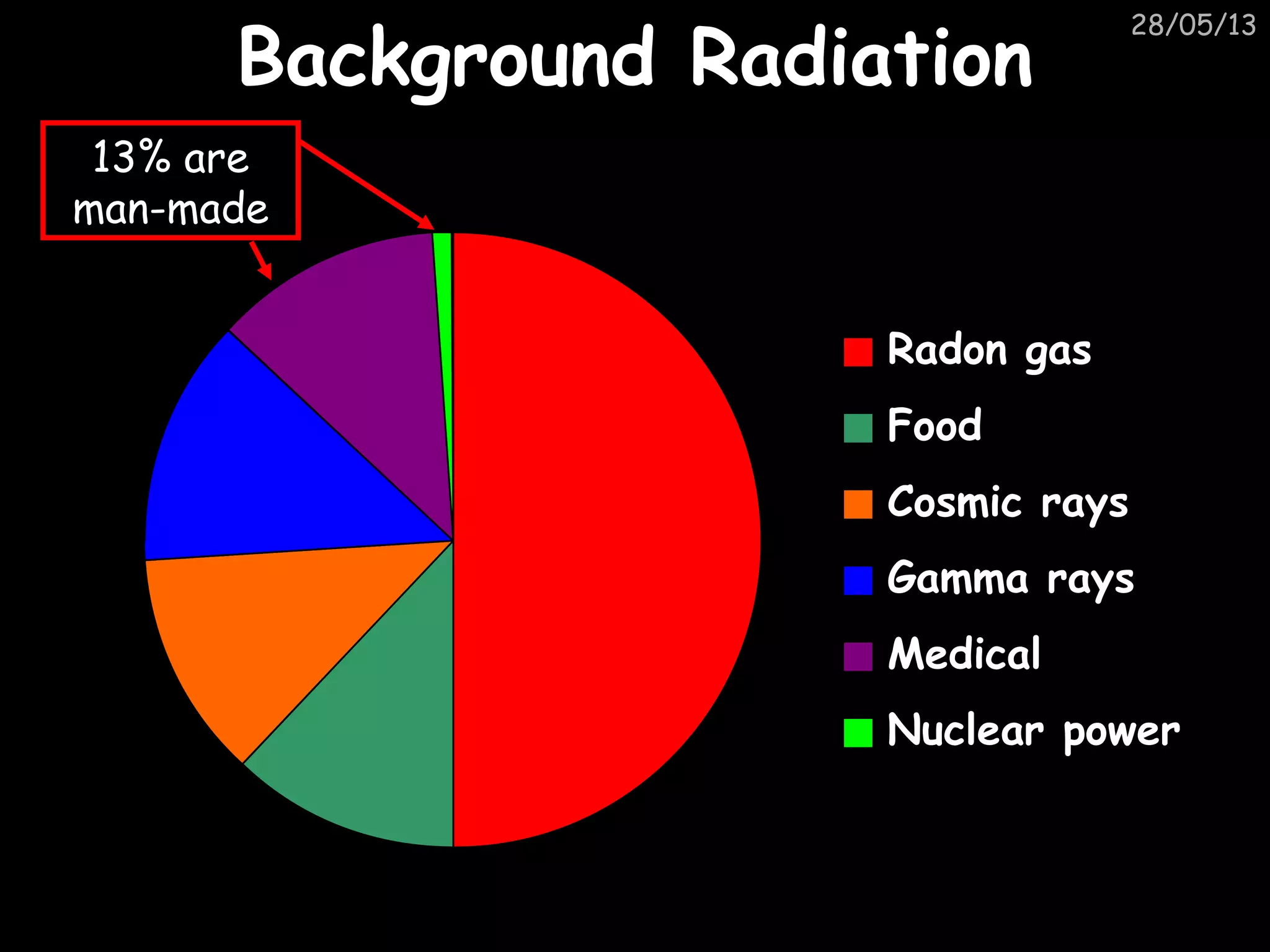
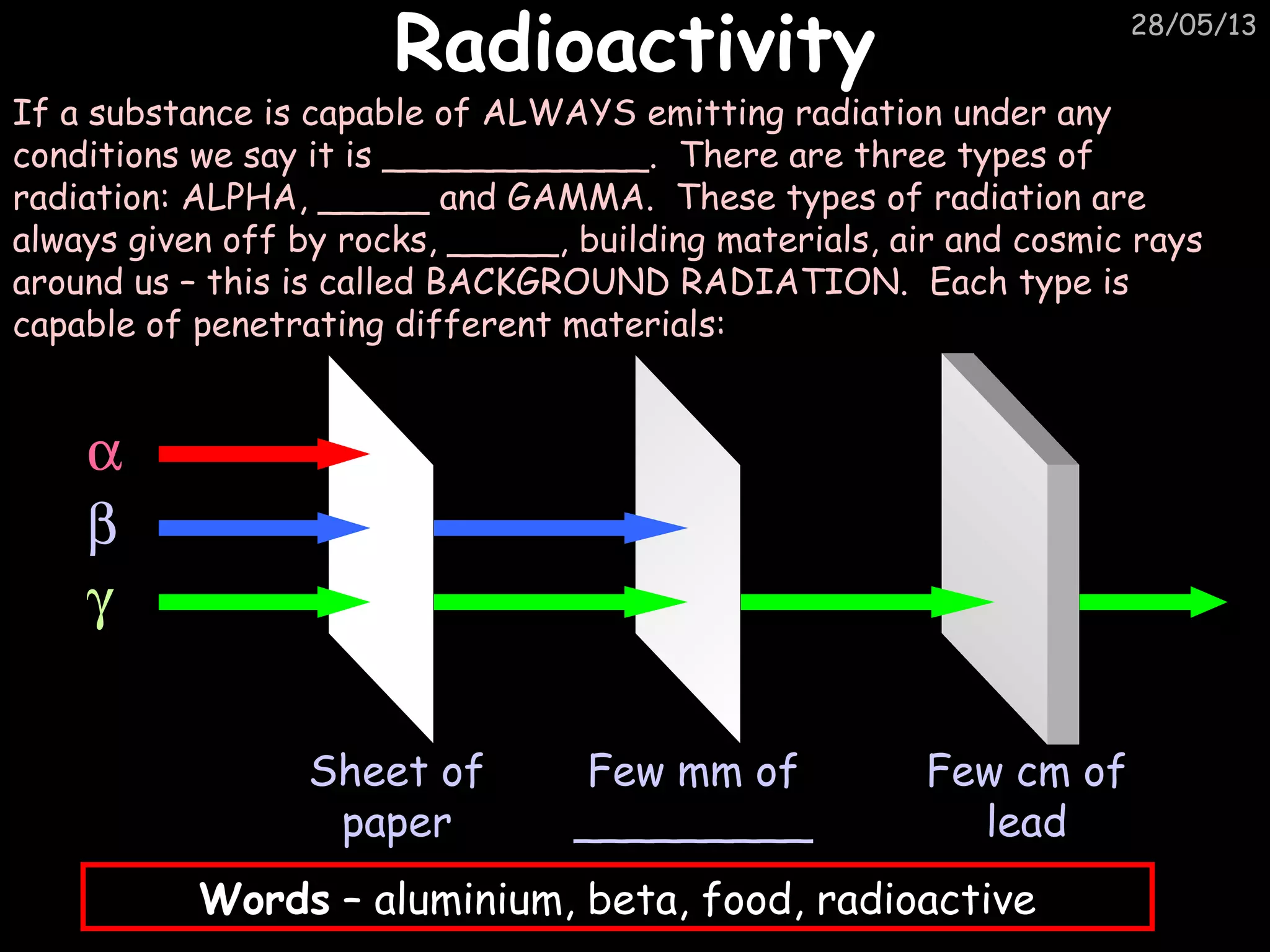
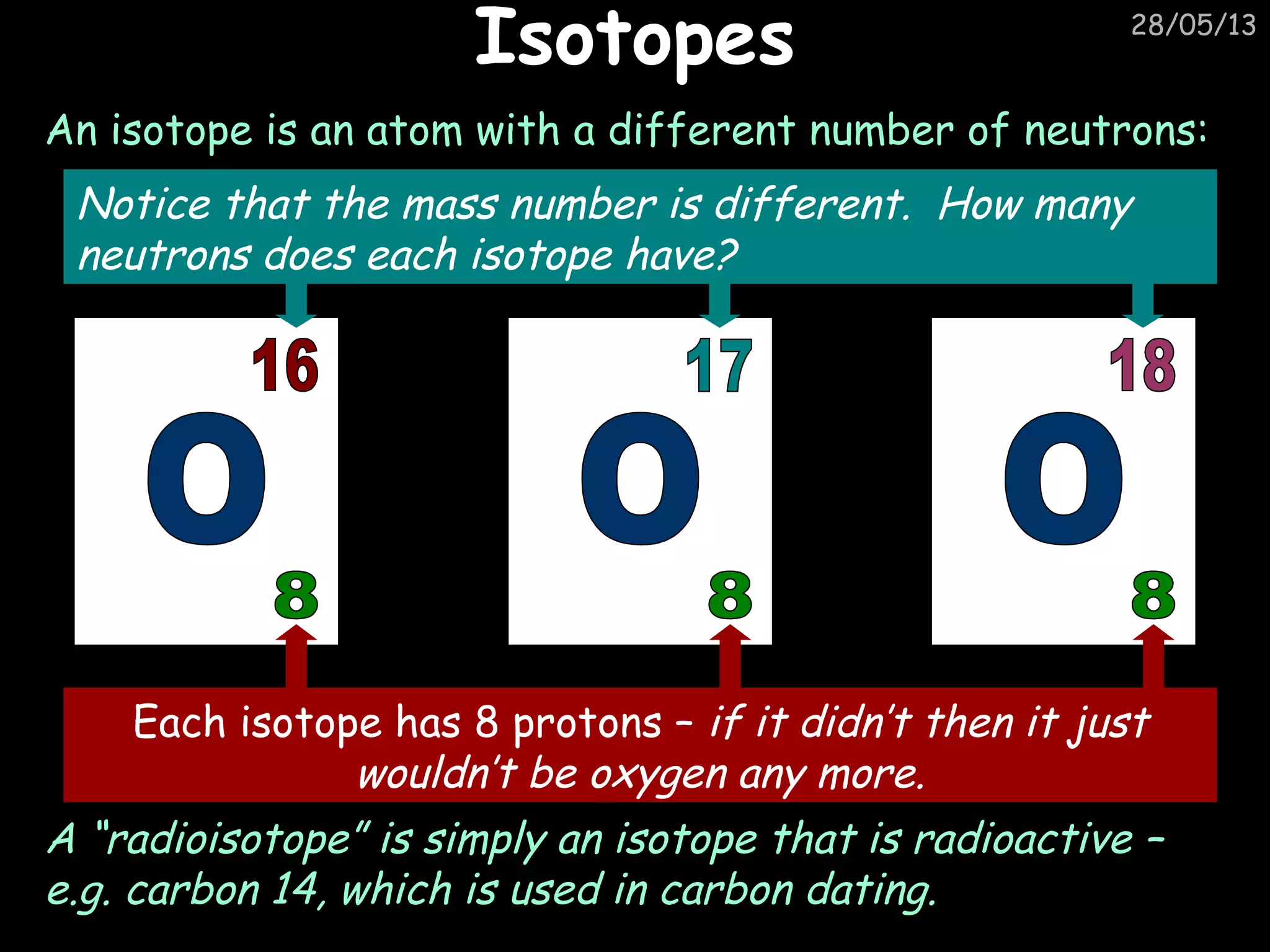
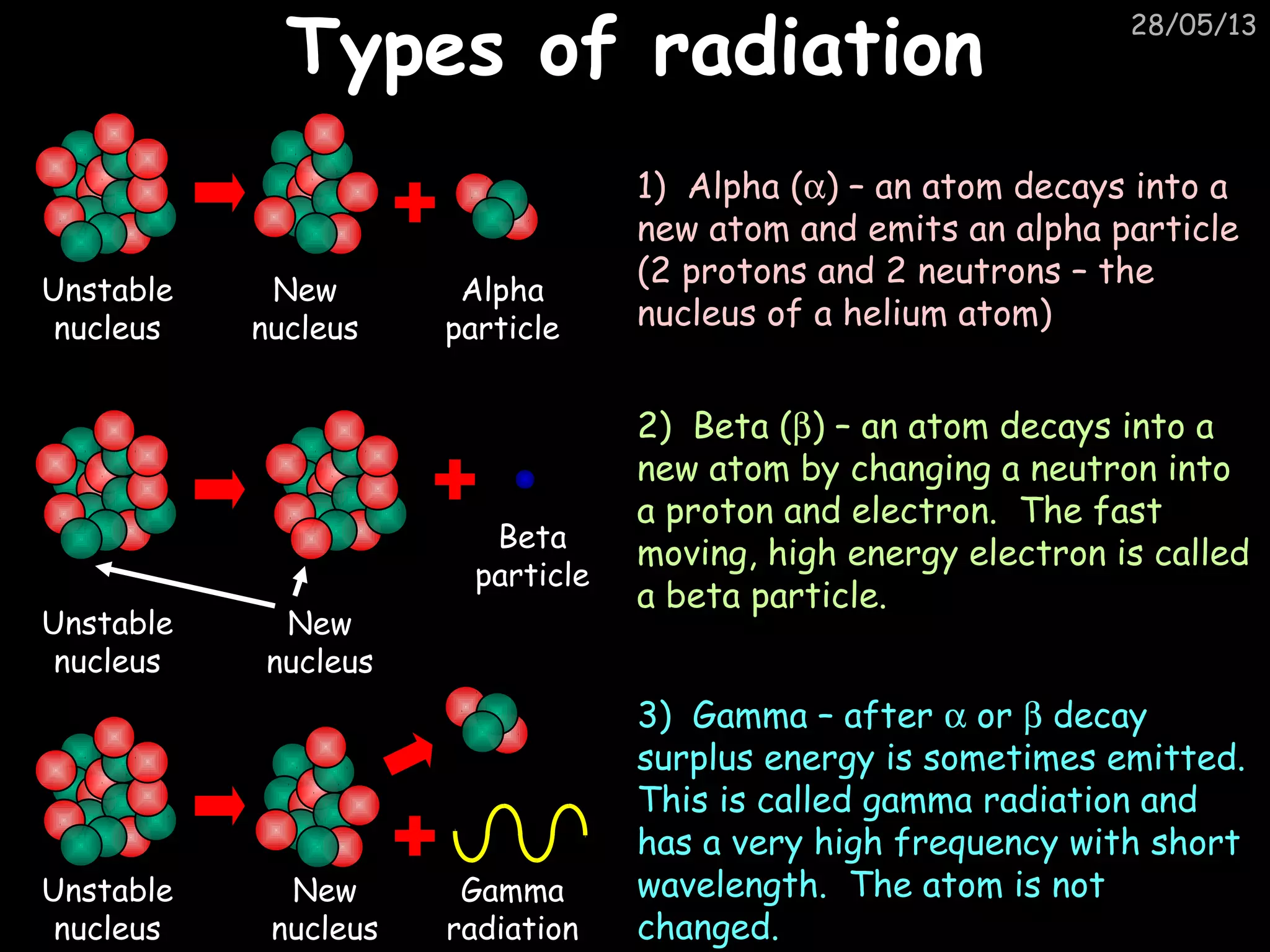
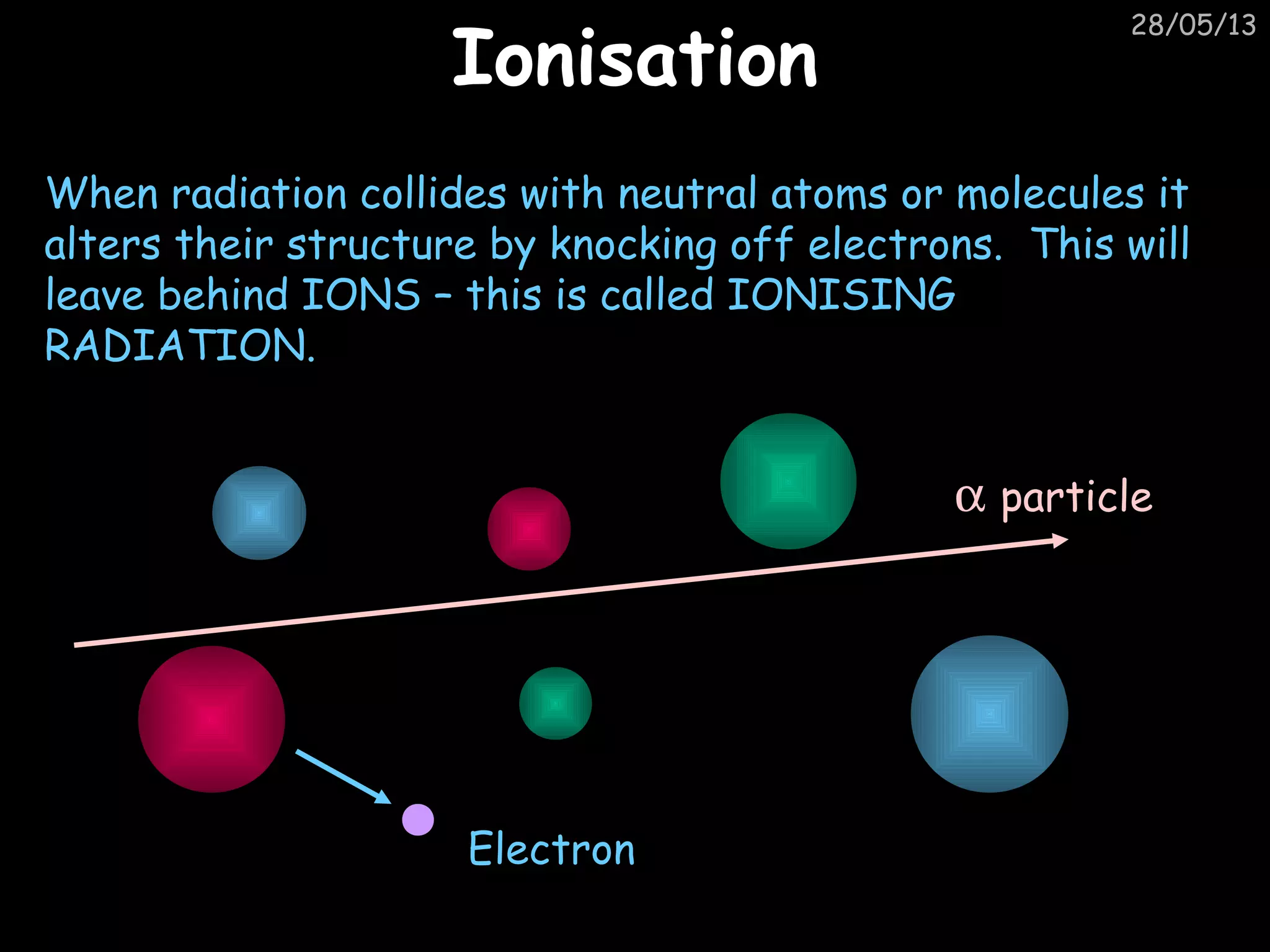
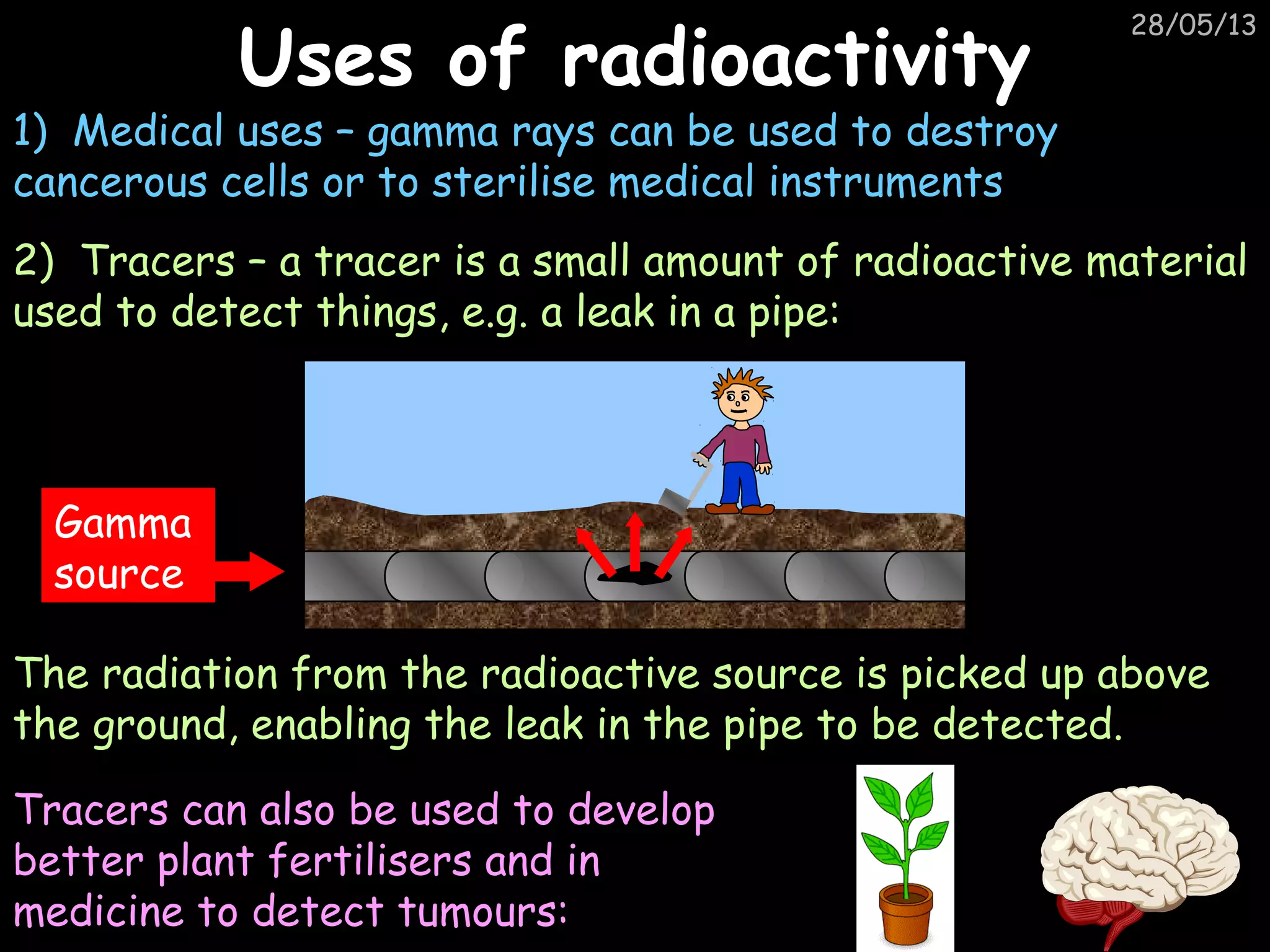
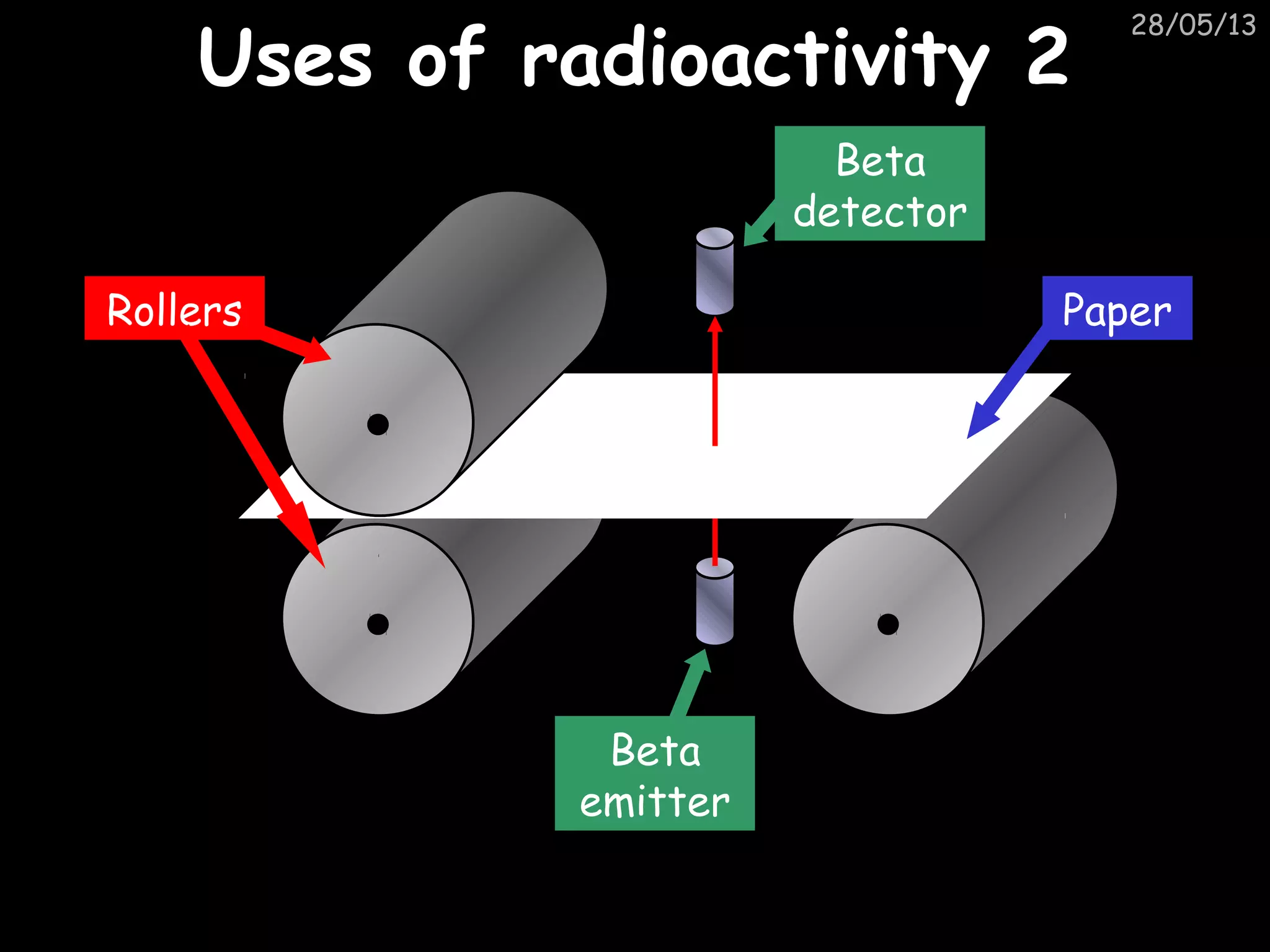
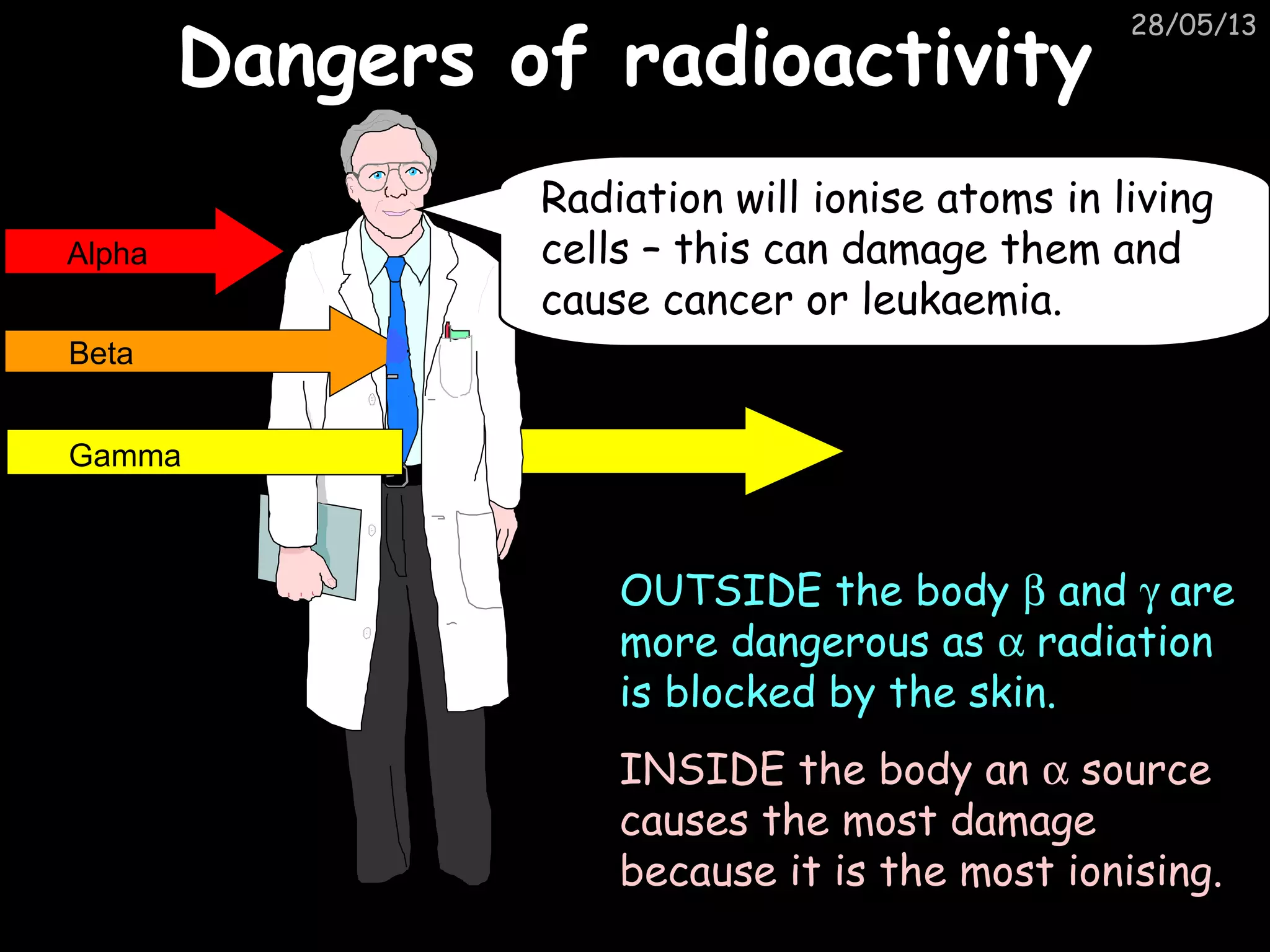
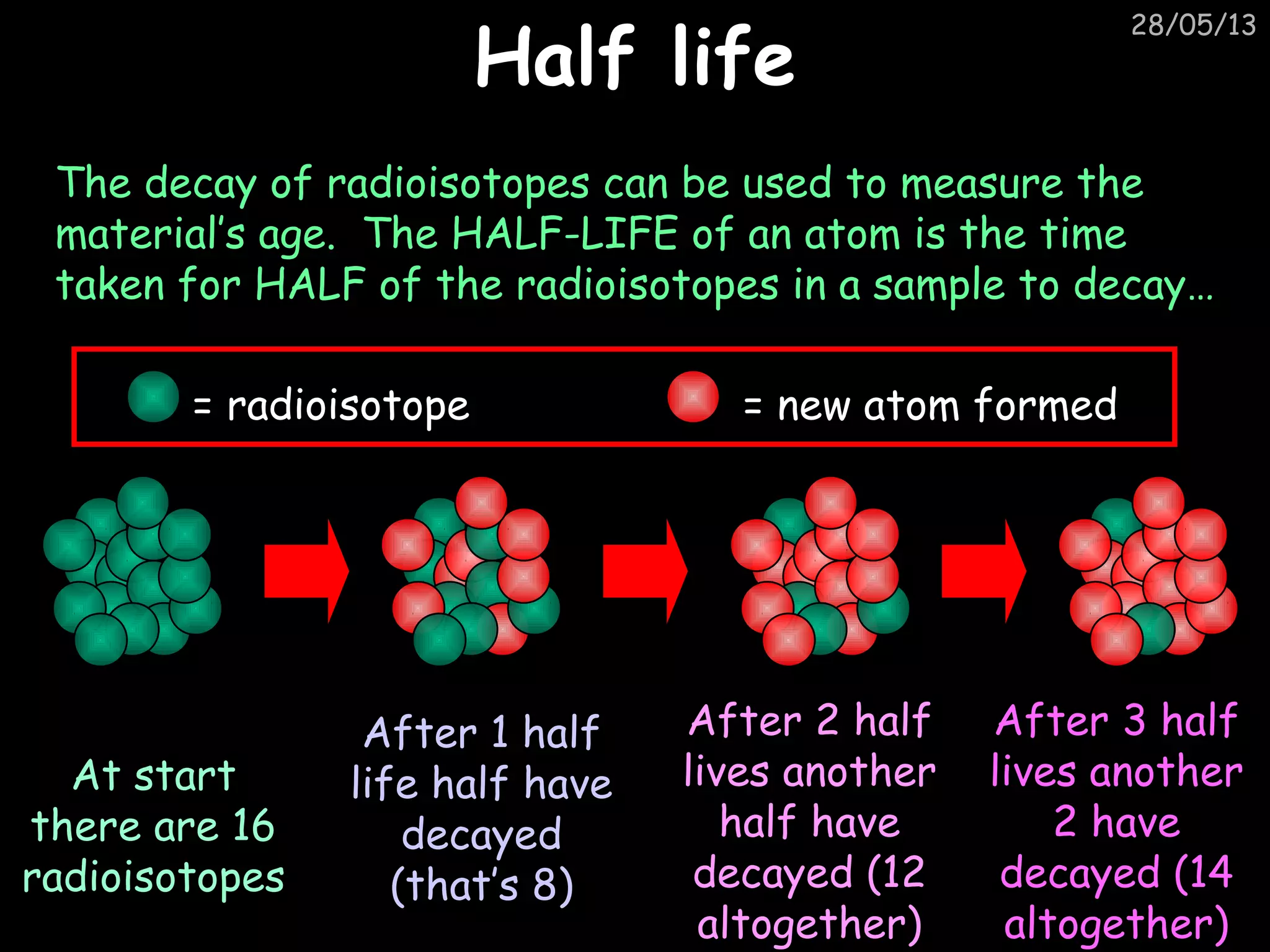
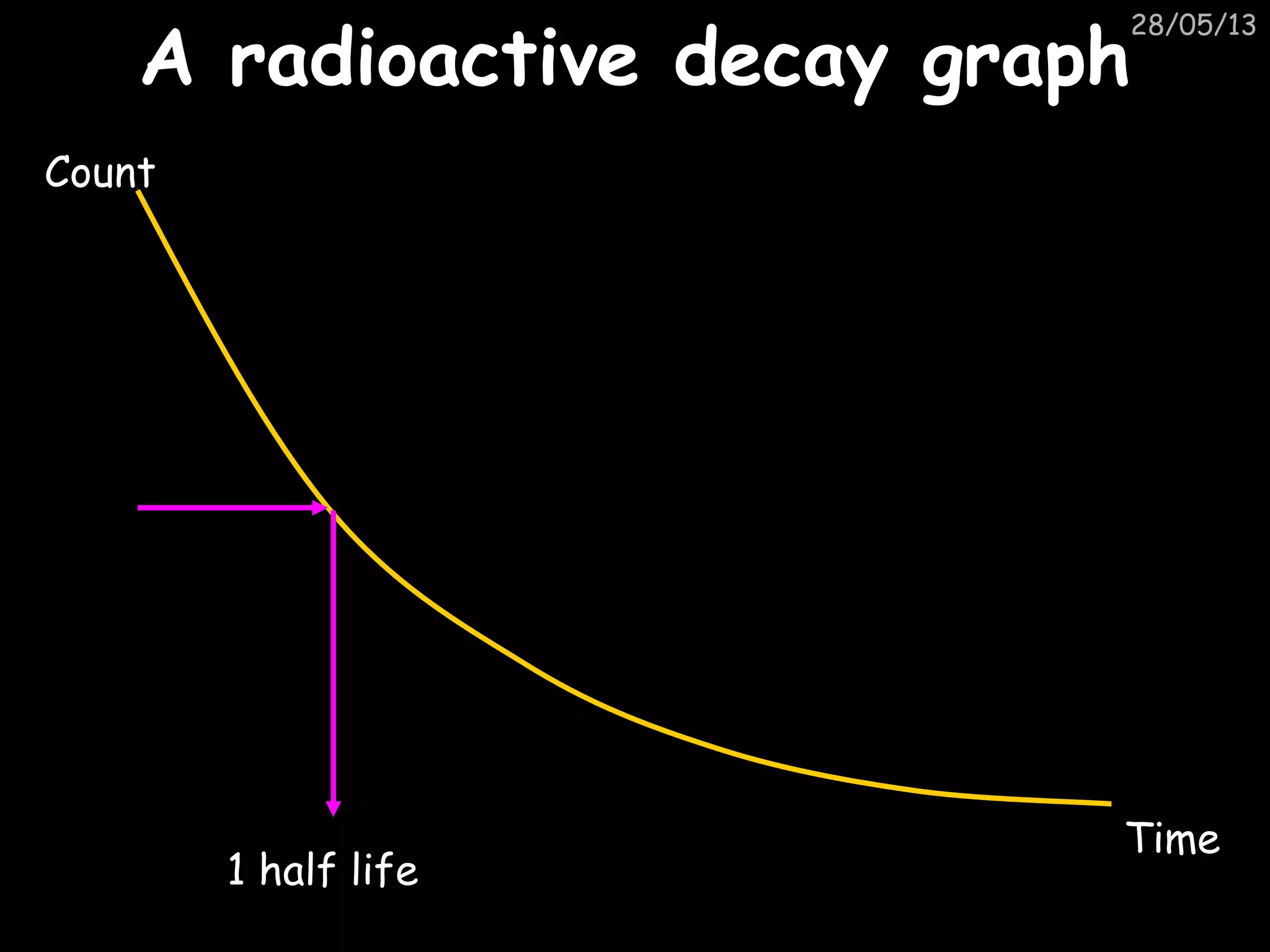
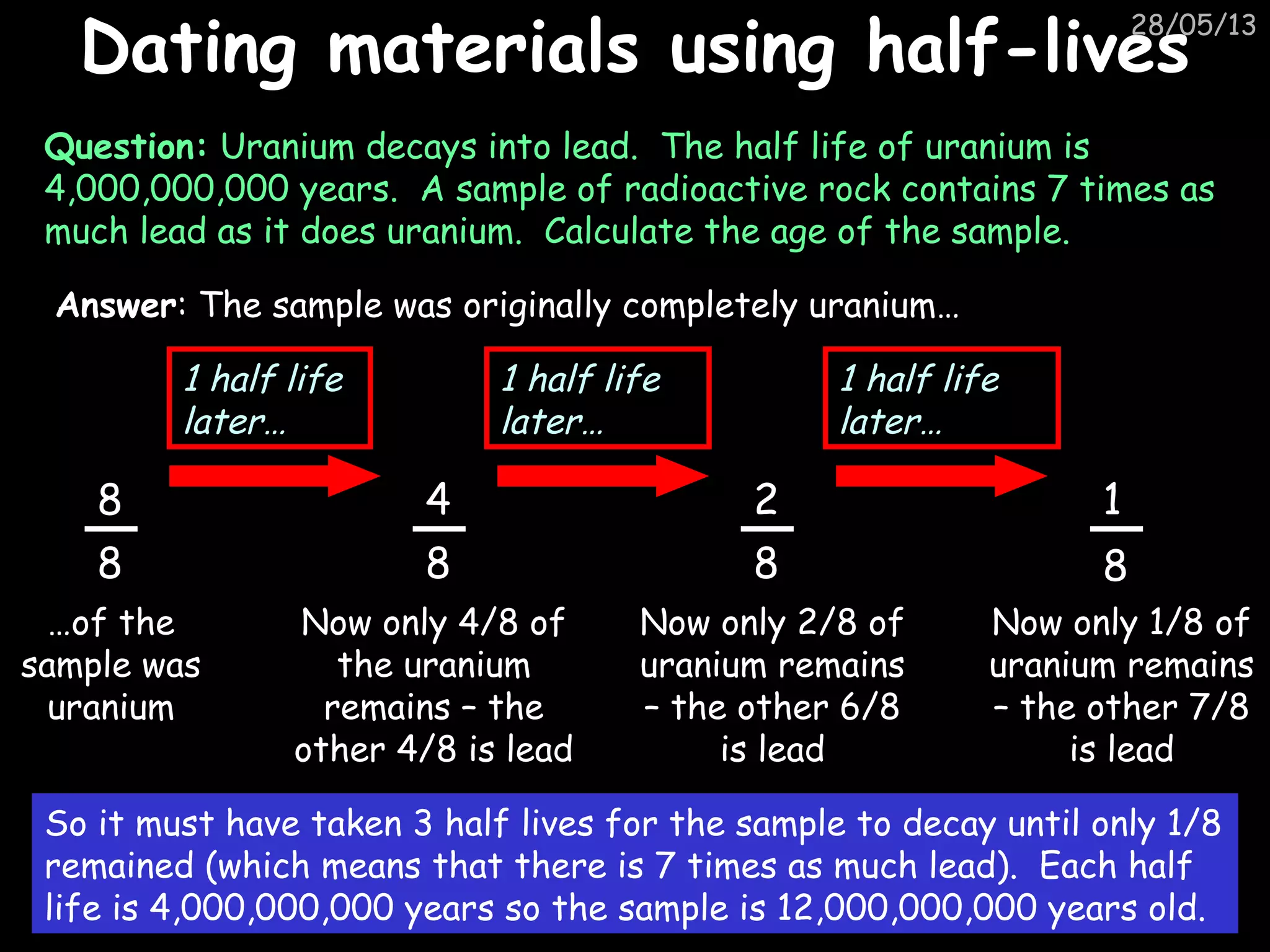
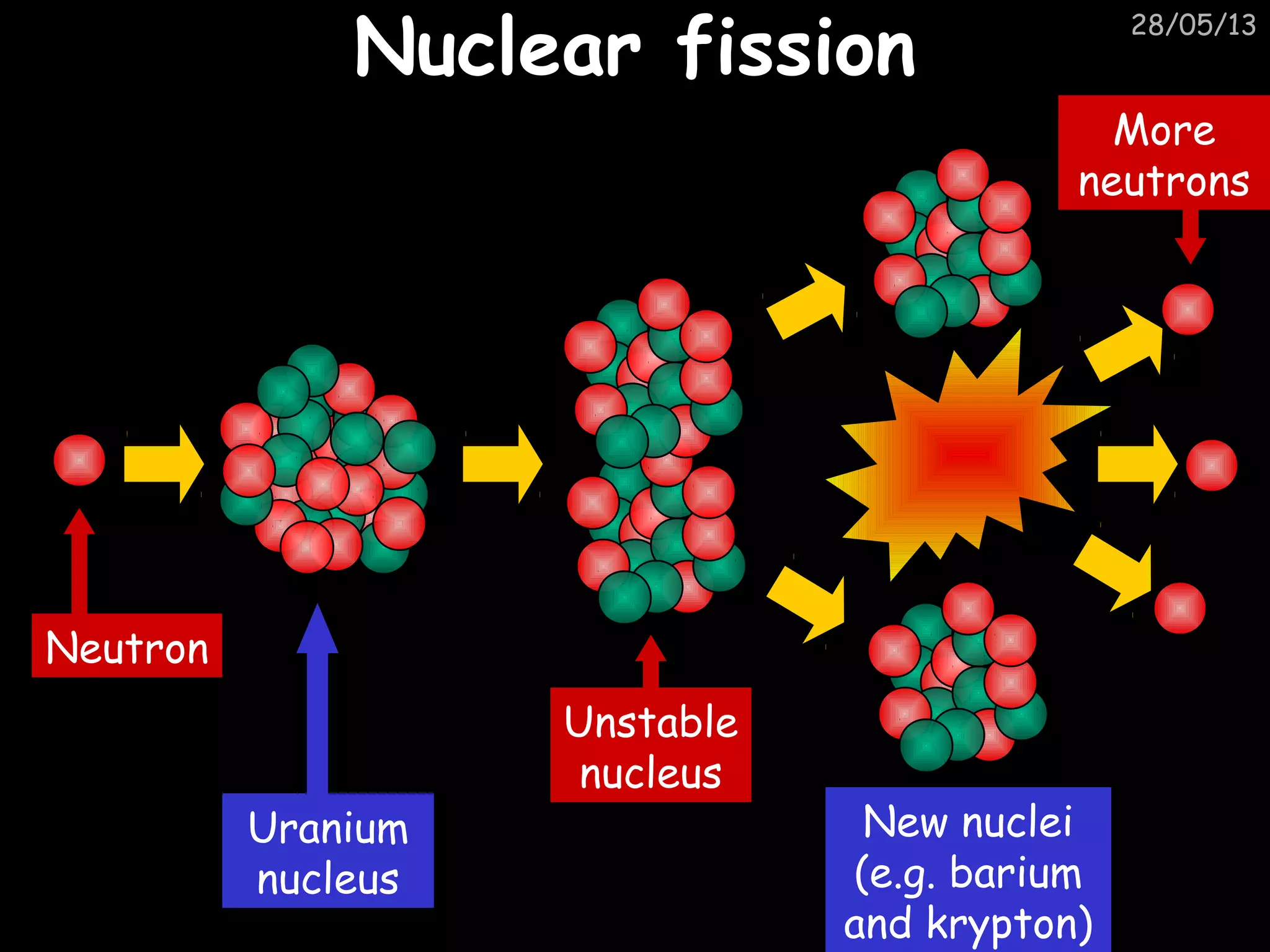
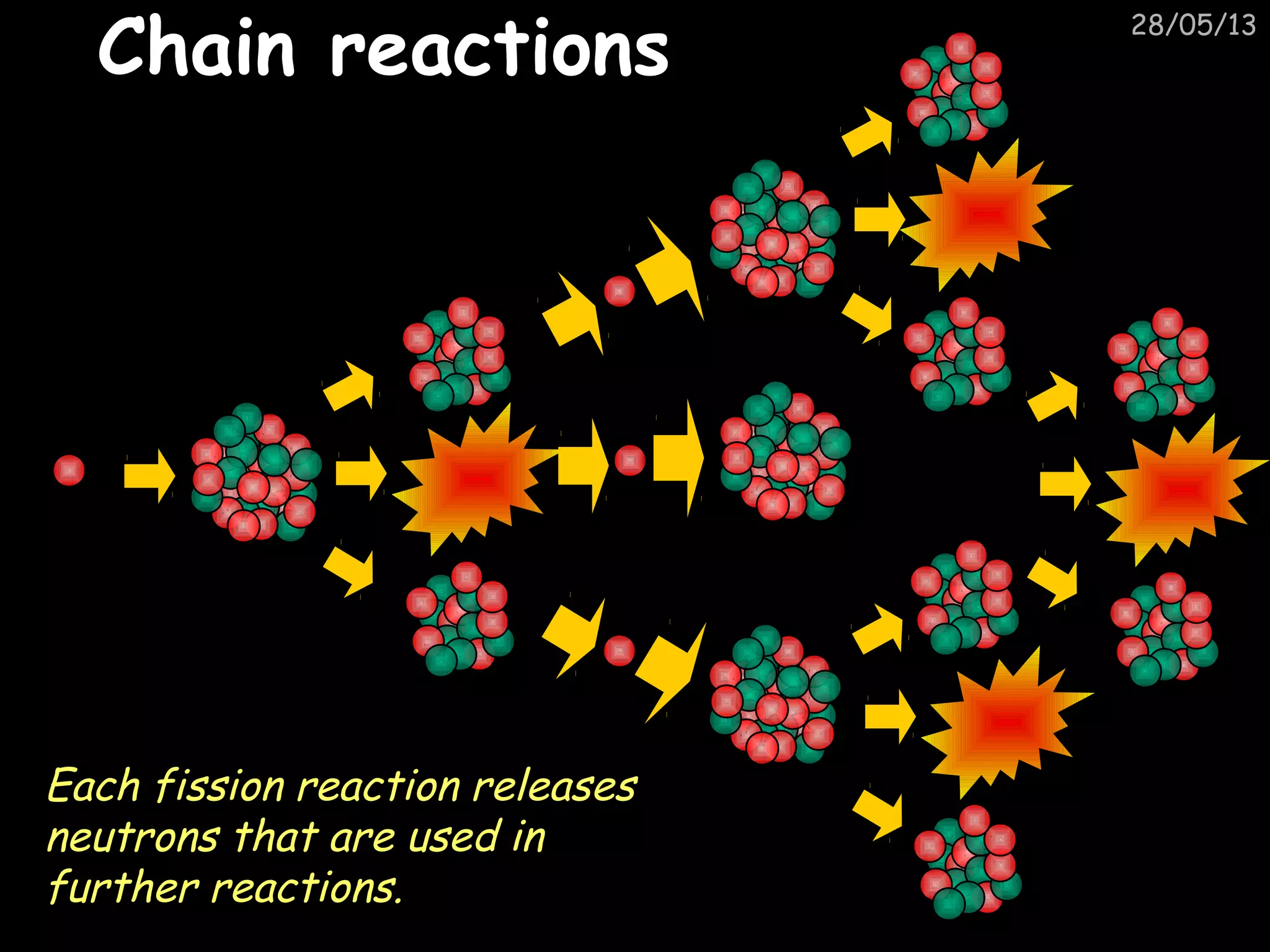
The document discusses the structure of atoms and types of radiation. It describes Ernest Rutherford's scattering experiment which proved that atoms have a small, dense nucleus surrounded by electrons. There are three types of radiation - alpha, beta, and gamma - which differ in their penetrating ability. Radioactive decay and half-lives are explained, showing how radioisotopes can be used to date materials. Nuclear fission is summarized as the splitting of uranium nuclei which releases neutrons and causes a chain reaction.