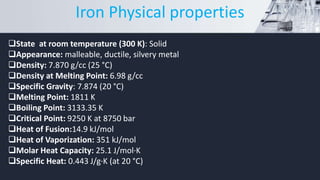
This document summarizes information about the chemical element iron. It discusses iron's history, noting that it is one of the earliest and most abundant elements on Earth. It then covers iron's physical properties such as its metallic appearance and high melting point. The document also examines iron's chemical properties, including its reactivity with oxygen and ability to form compounds. Additionally, it explores iron's magnetic properties below the Curie point and its many applications, such as in construction and electronics. The summary concludes by mentioning iron's essential biological role in blood production and oxygen transport.