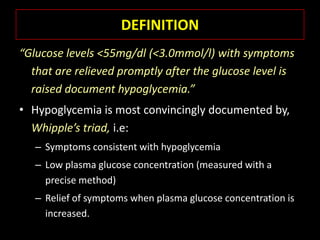
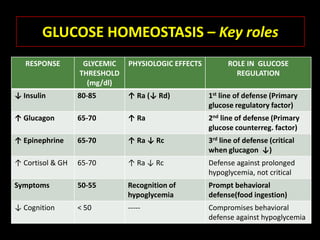
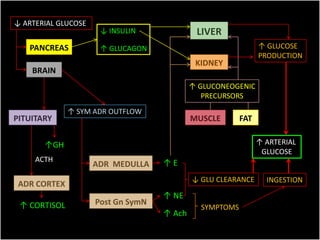
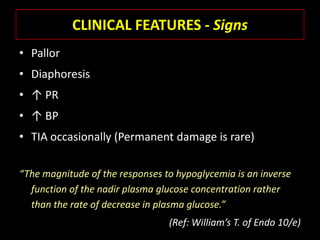
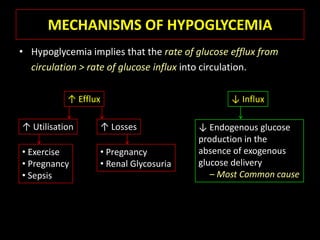
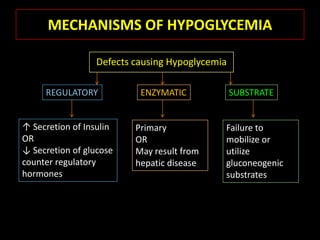
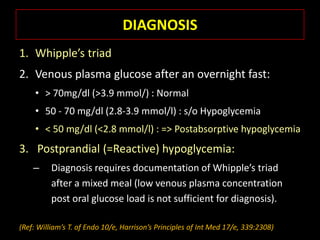
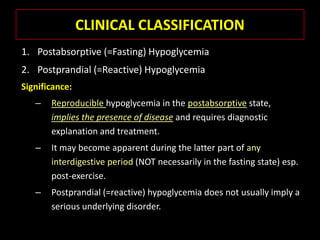
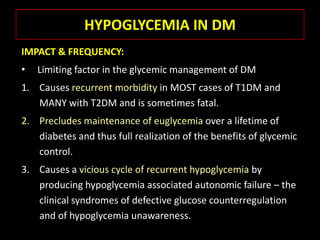
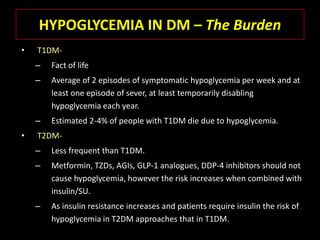
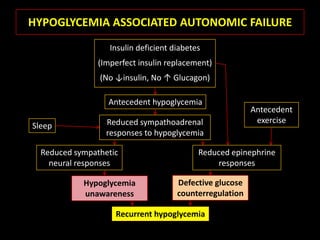
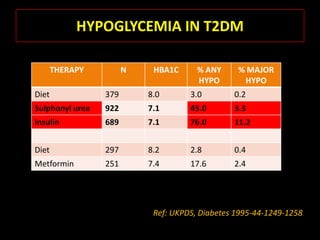
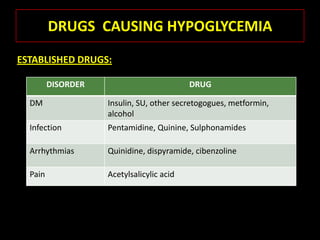
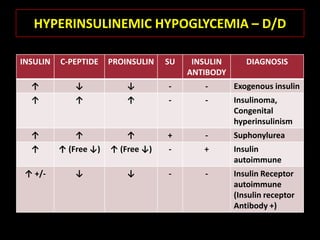
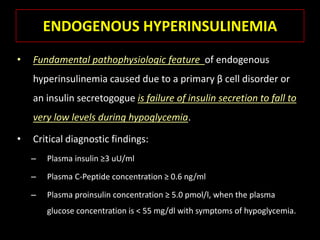
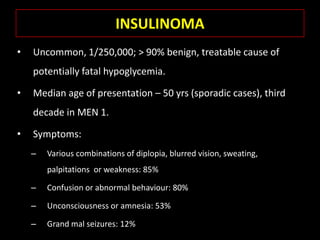
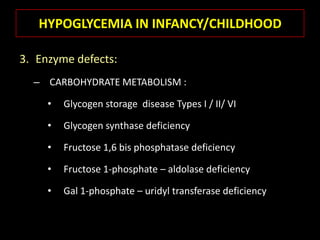
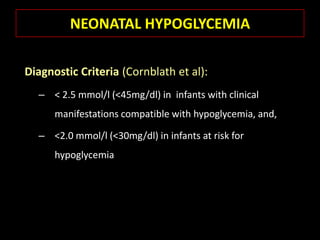
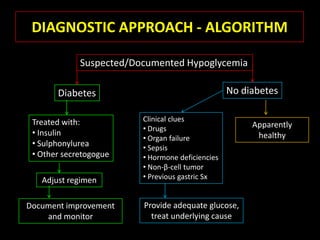
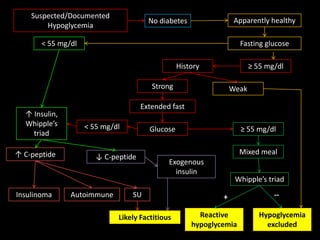
This document summarizes key points about hypoglycemia and diabetic emergencies. It defines hypoglycemia and describes glucose homeostasis and the body's response to low blood sugar levels. The clinical features and mechanisms of hypoglycemia are outlined. Hypoglycemia is classified as either postabsorptive or postprandial. Postabsorptive hypoglycemia implies an underlying disease that requires diagnosis and treatment. Hypoglycemia is a major problem for patients with diabetes and predisposes them to recurrent low blood sugar episodes through hypoglycemia-associated autonomic failure. Conventional risk factors are based on relative or absolute insulin excess compromising the body's natural defenses against dropping glucose levels.