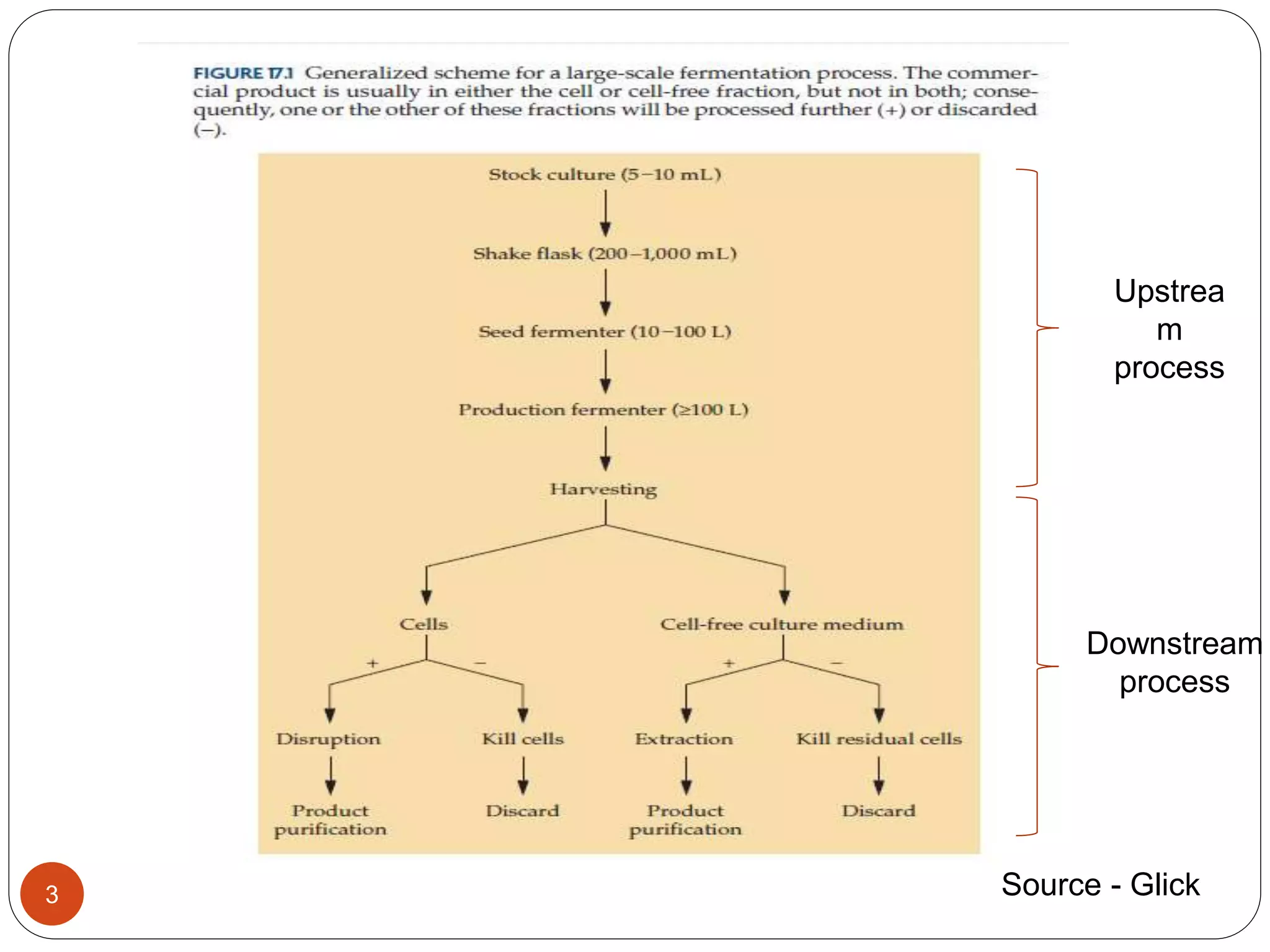
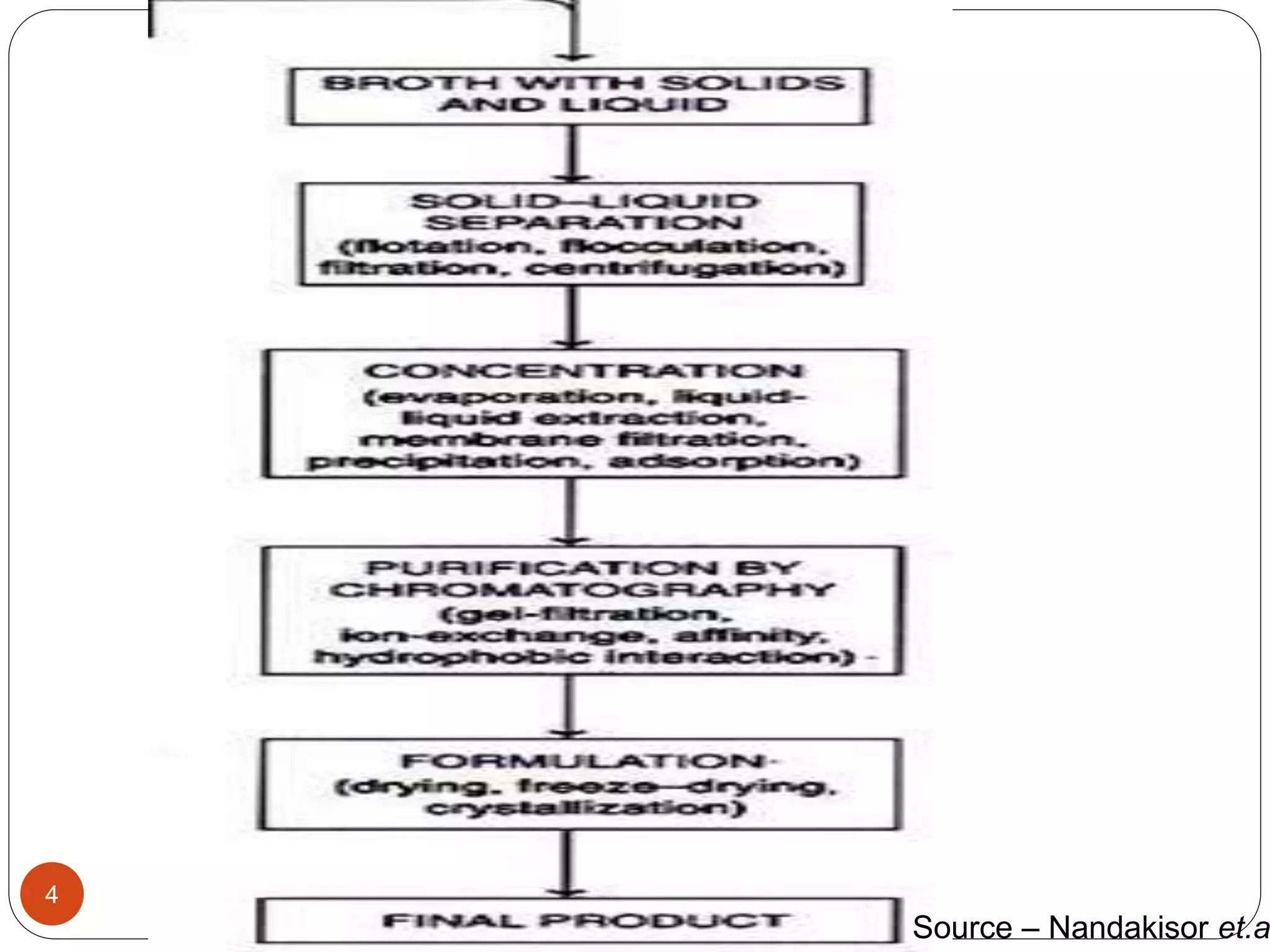
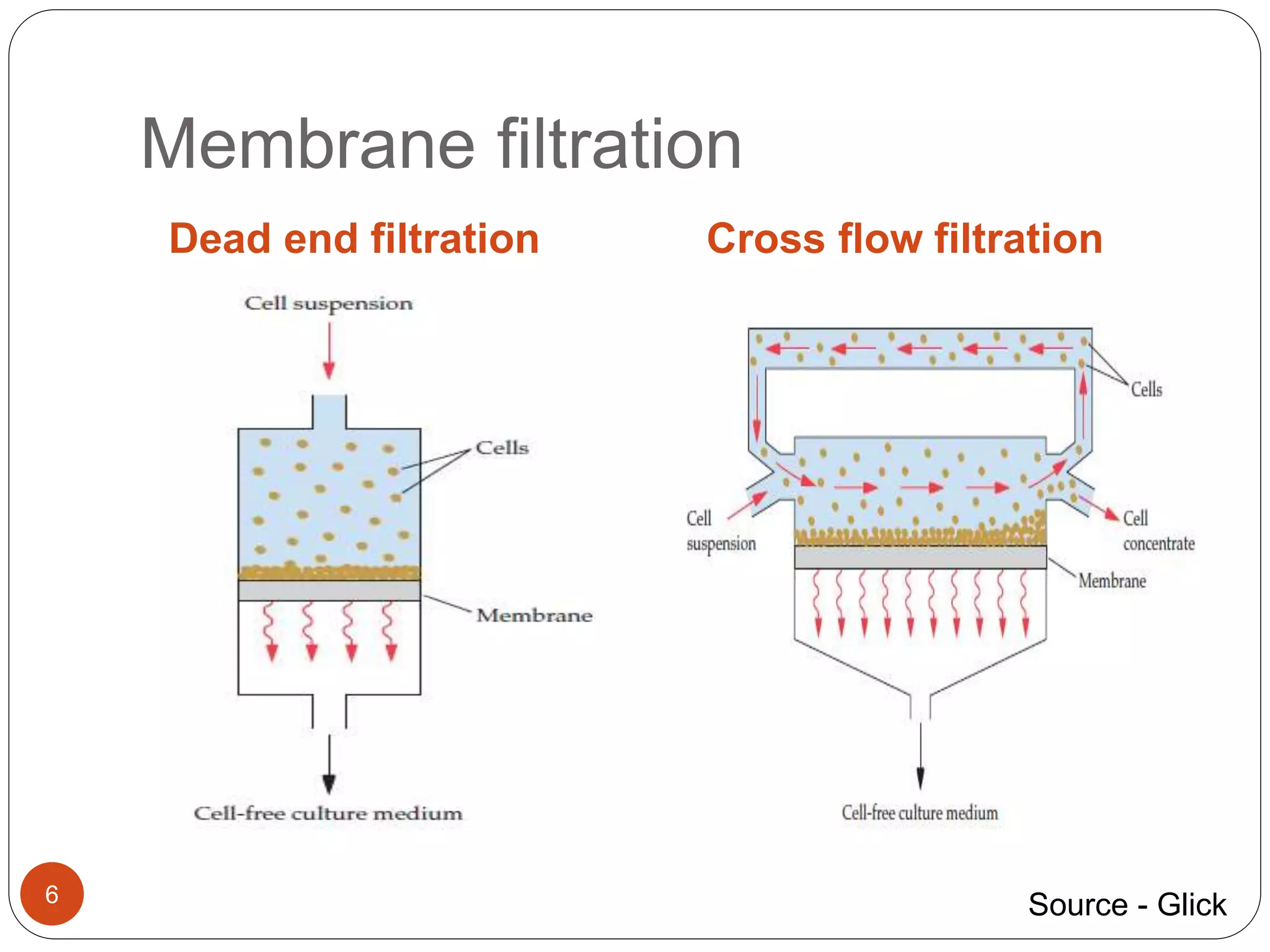
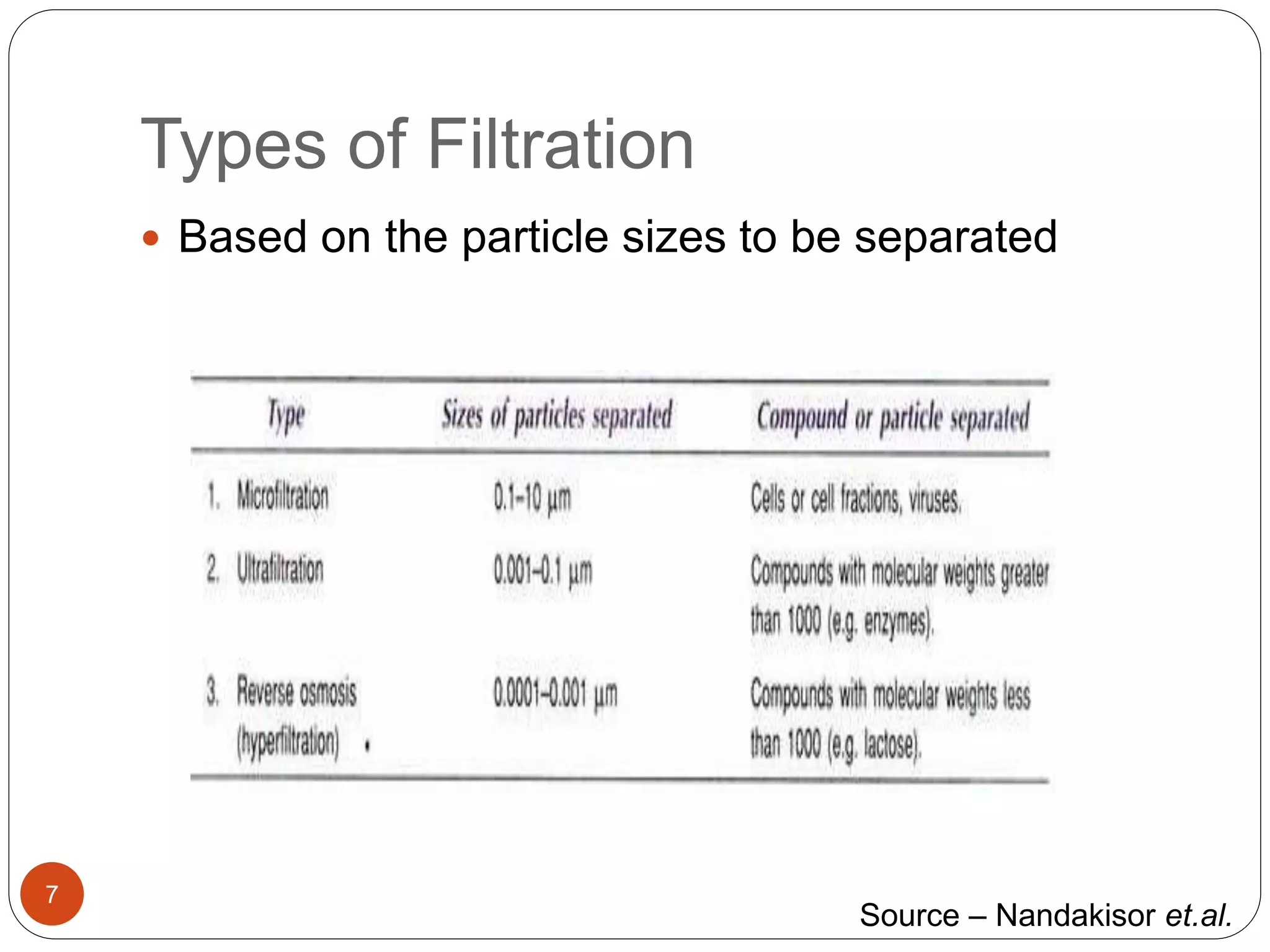
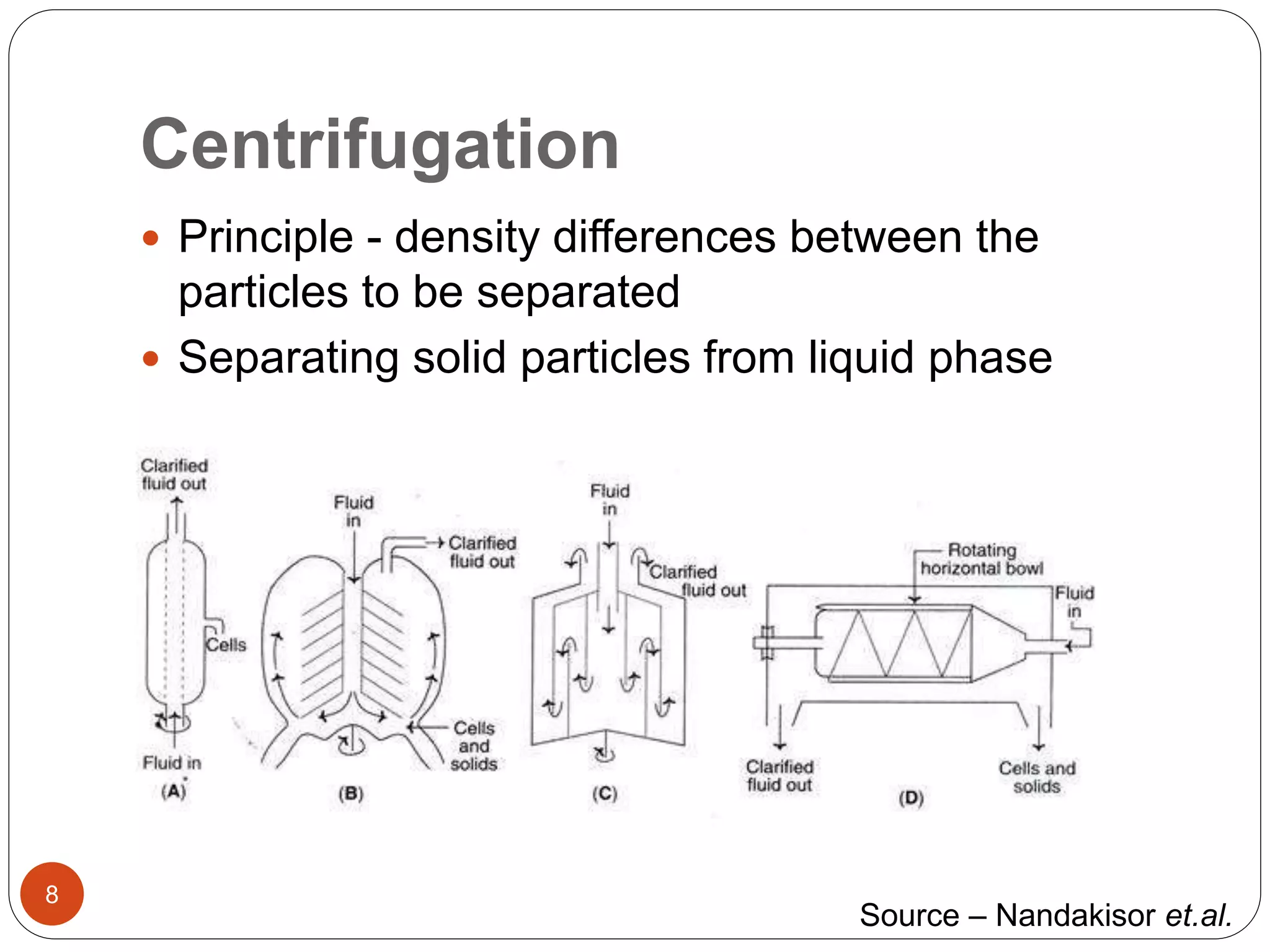
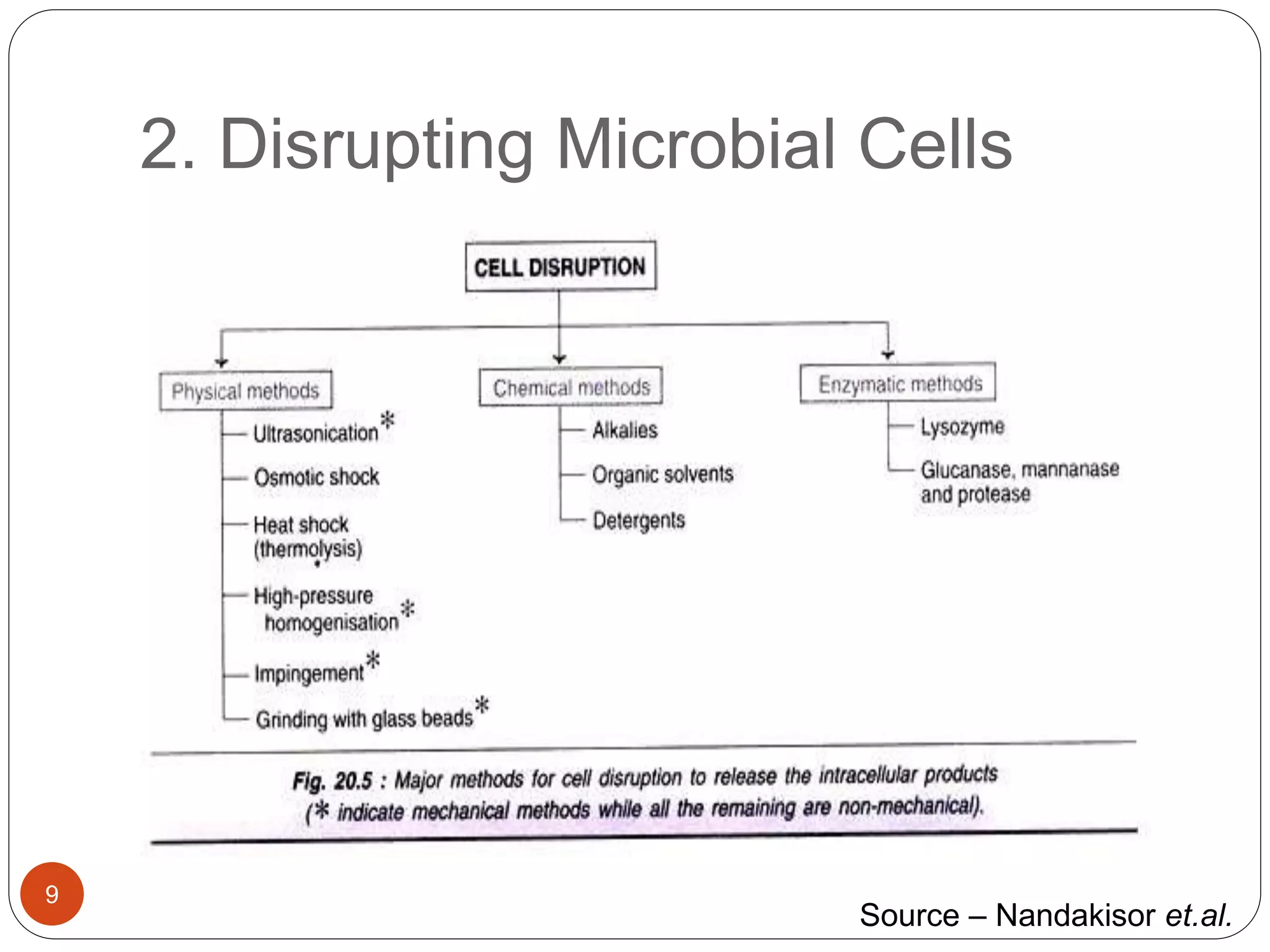
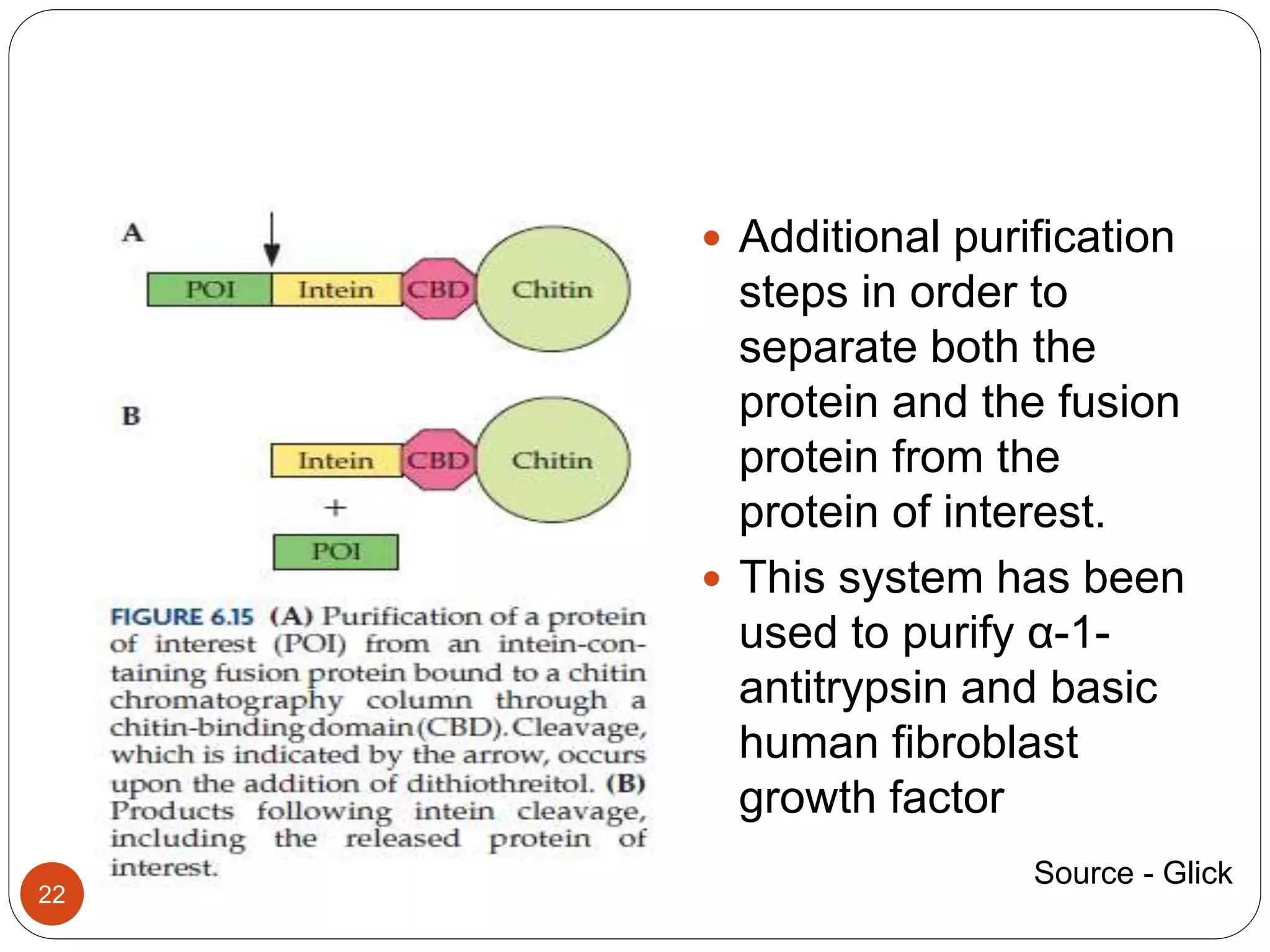
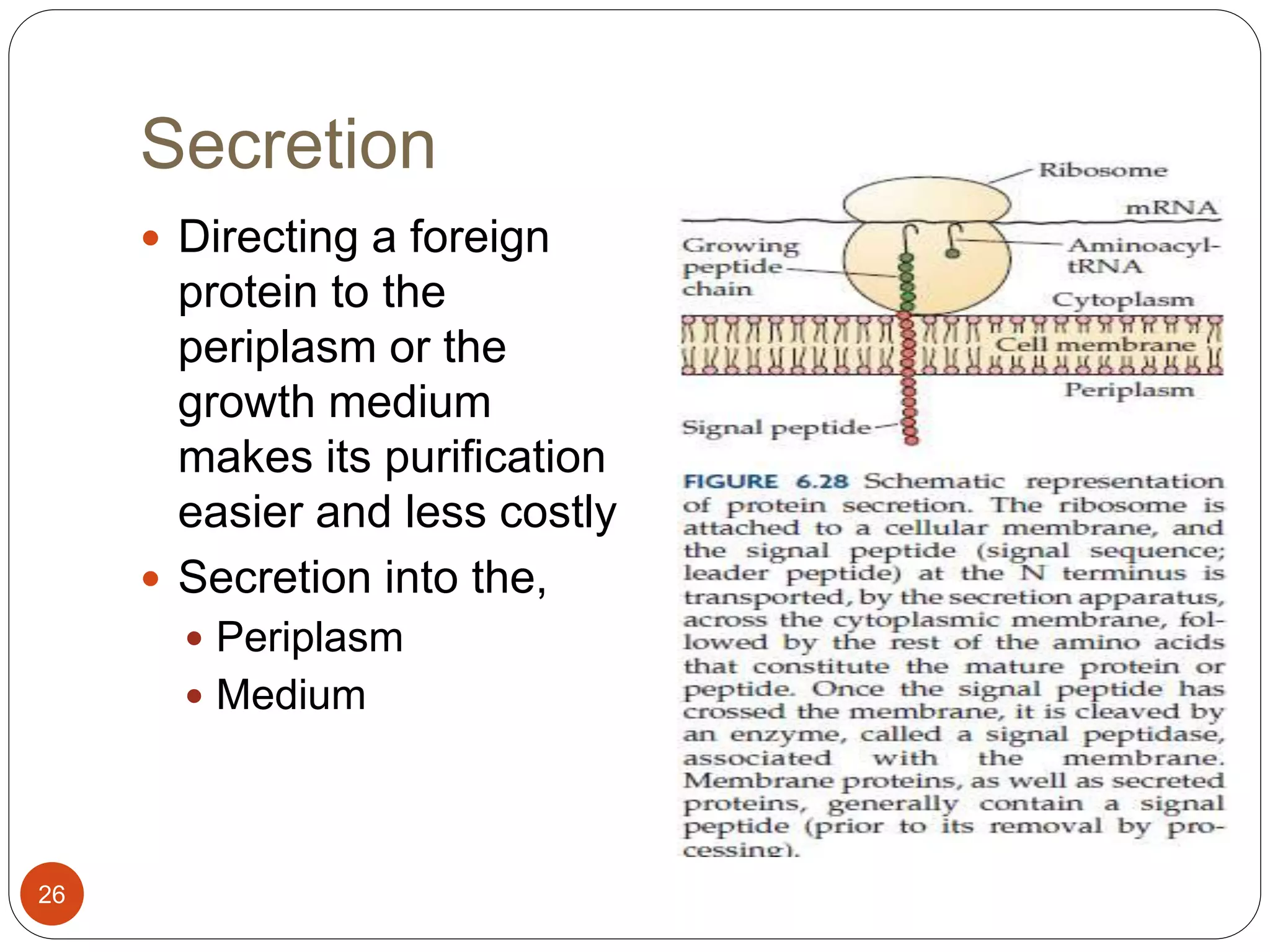
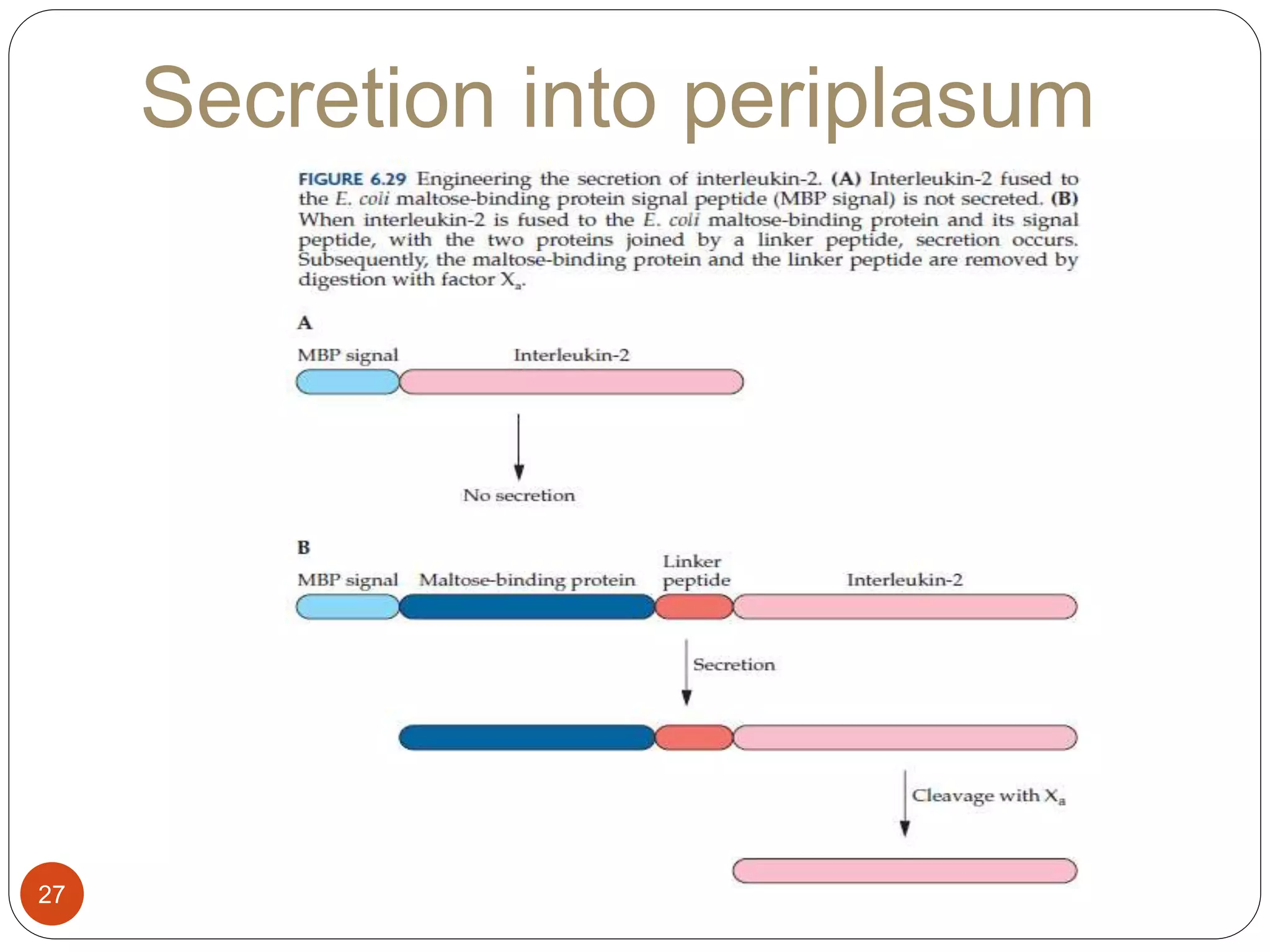
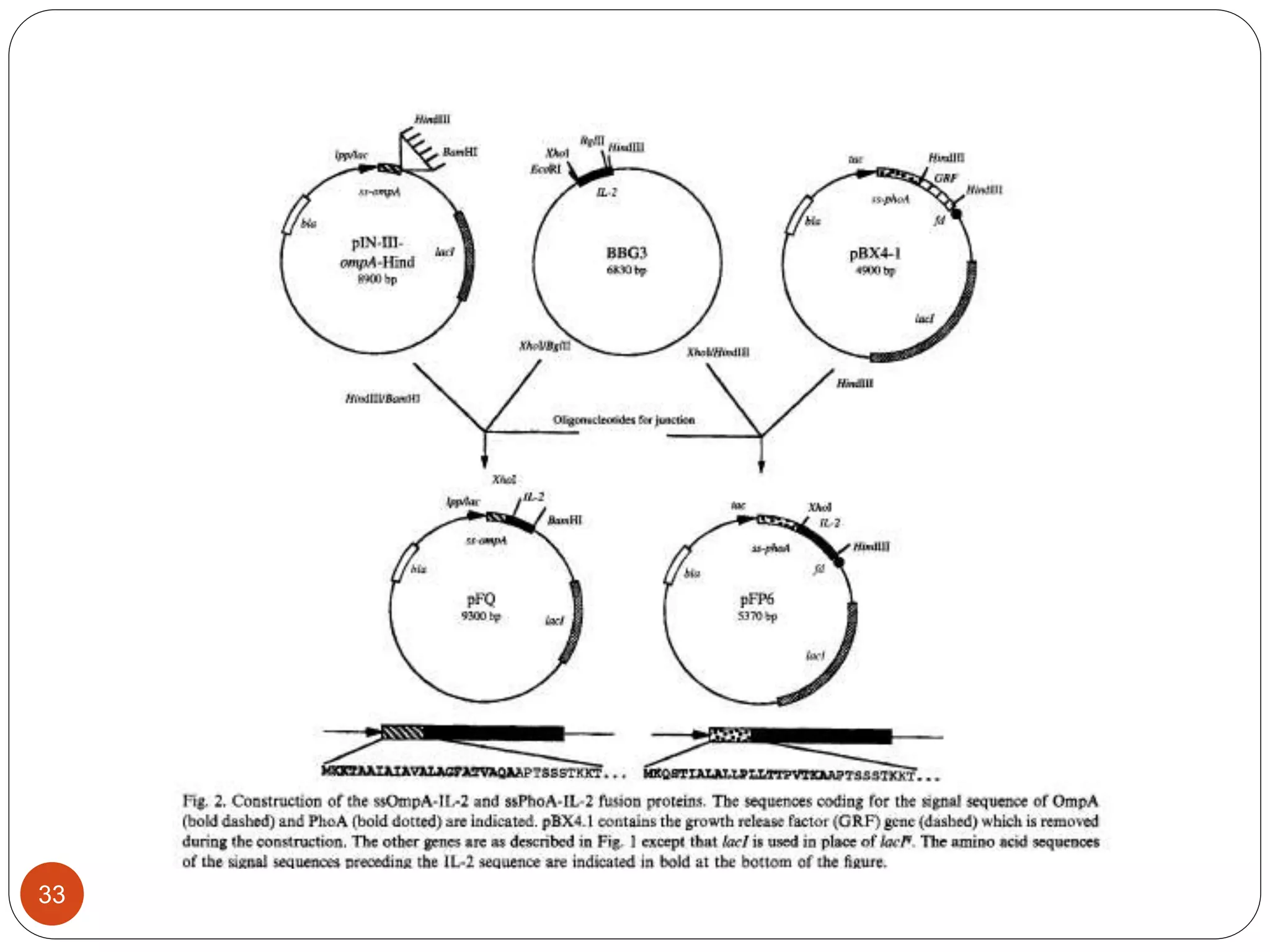
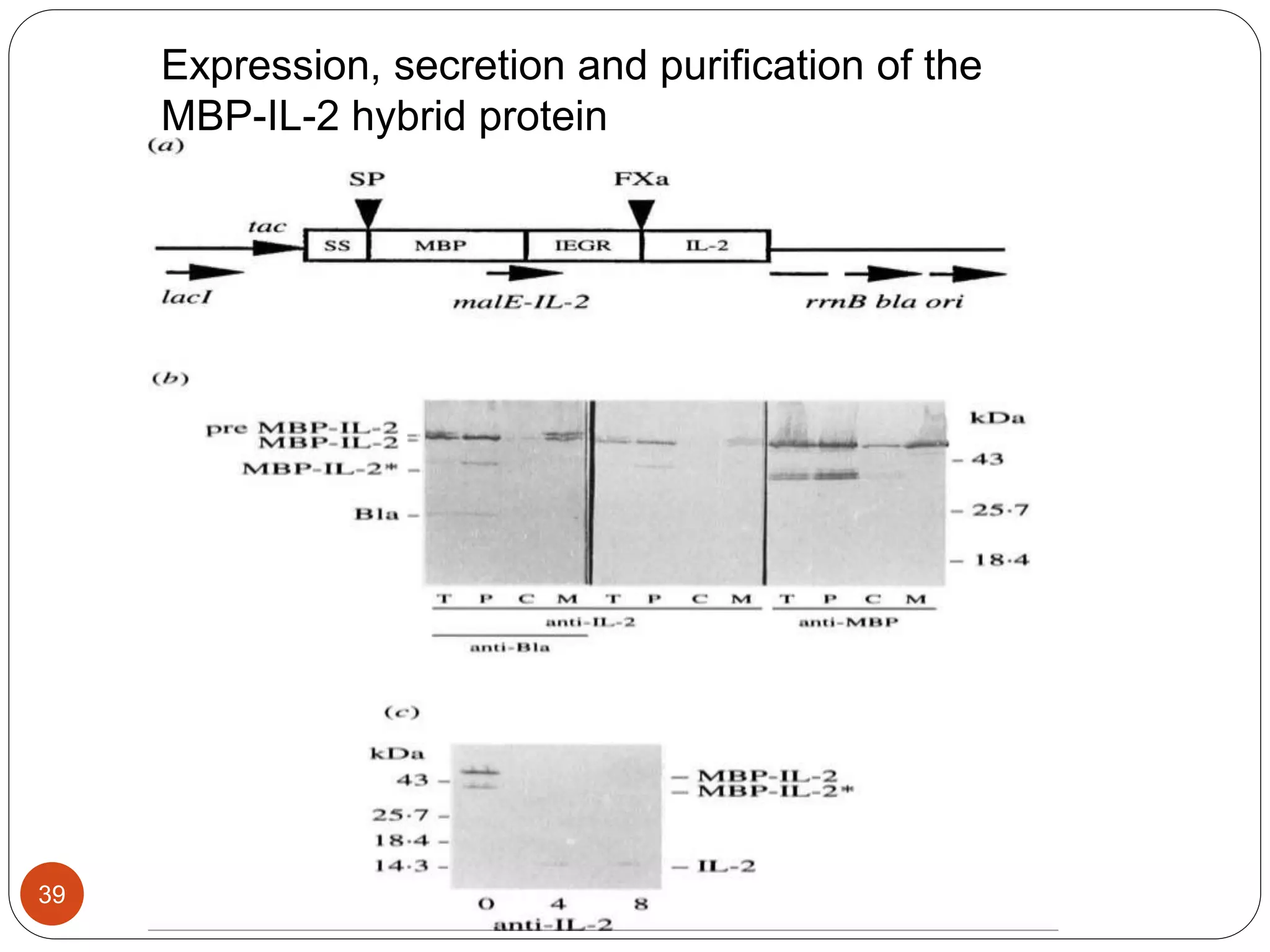
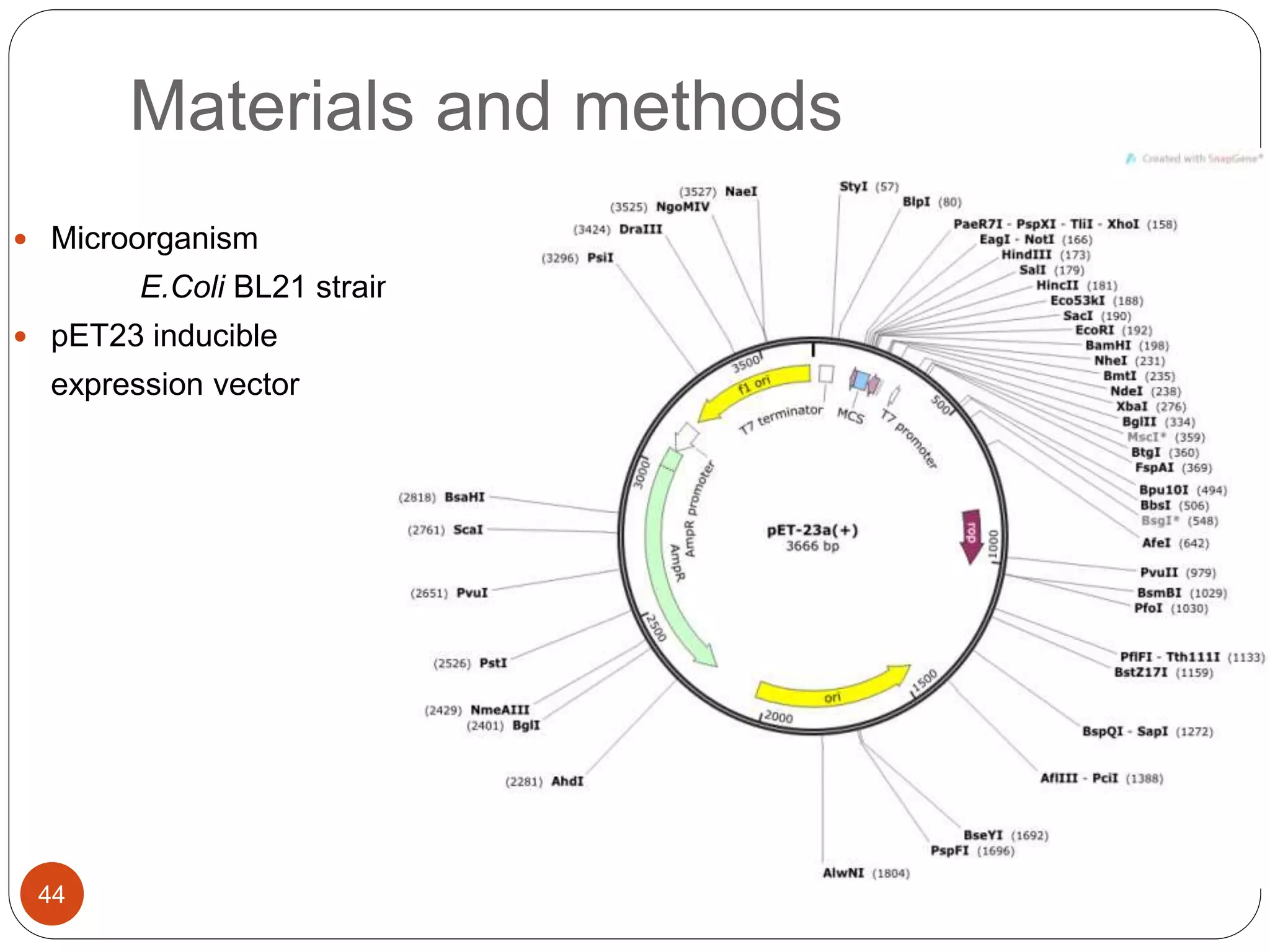
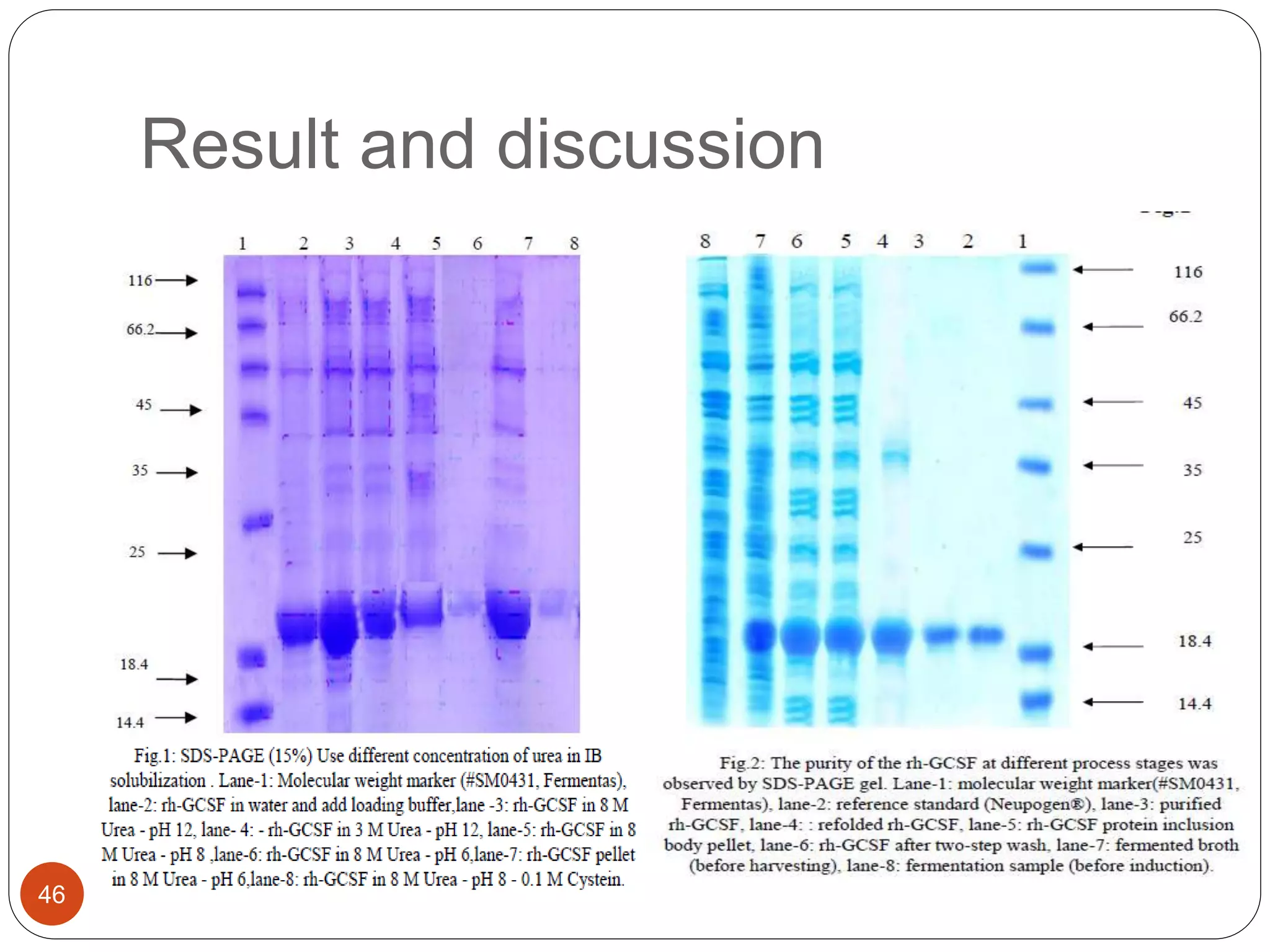
The document discusses downstream processing (DSP) in biotechnology, which is critical for extracting and purifying products from microbial fermentation, particularly in pharmaceuticals. It covers various DSP techniques, such as cell harvesting, cell disruption, concentration, chromatography, and the issues surrounding protein stability and purification. Additionally, it highlights methods for improving separation and recovery of proteins, particularly recombinant human granulocyte colony-stimulating factor (rh-GCSF) from E. coli.